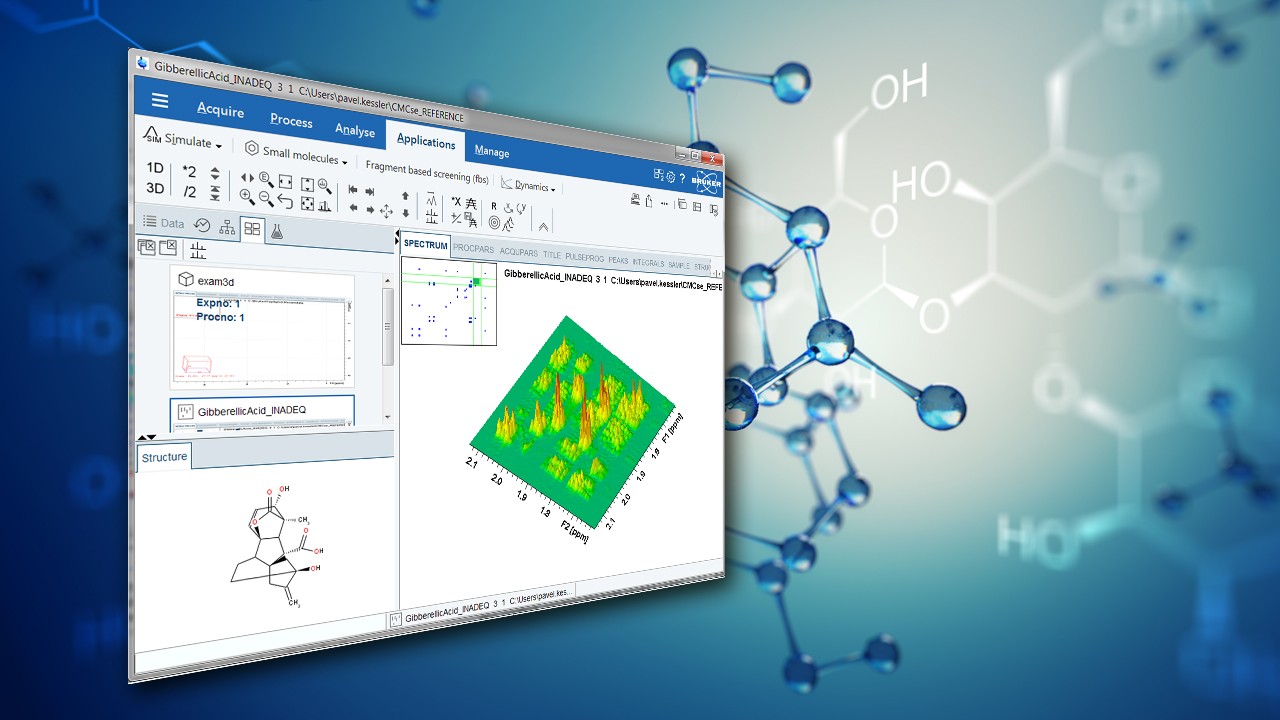
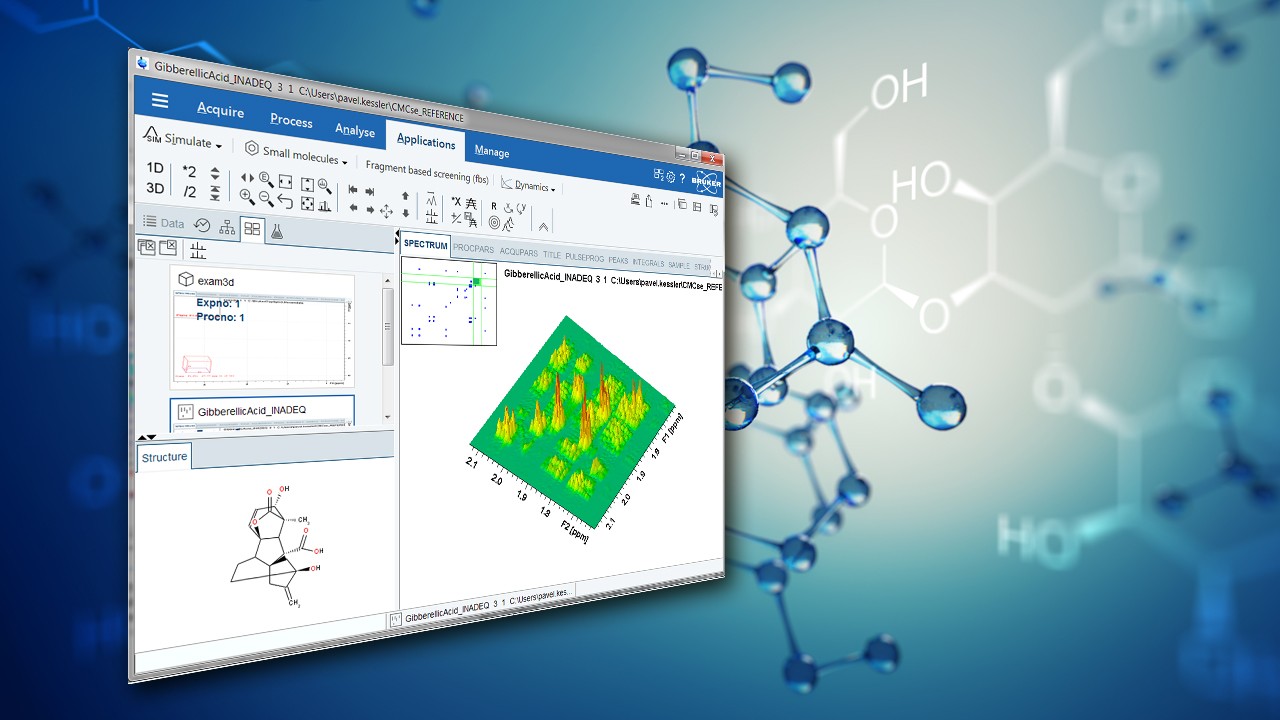
Fragment-based Screening Experiments in TopSpin
In a recent application note, Bruker applications scientist Donna Baldisseri outlines how to use TopSpin software for fragment-based screening.
Introduction
Fragment-Based Drug Design, FBDD, is a powerful search engine for early identification of molecular fragments that bind to disease relevant target proteins ultimately leading to drug development (1). Fragments of larger molecules encompassing widely varying chemical space can be used to quickly and cost effectively assess the ligandability of a target protein. NMR spectroscopy is uniquely suited for this process because it can directly and rapidly identify weakly binding fragments which, through chemical refinement, produce promising drug candidates. Unlike protein observe techniques such as SAR (2), ligand observe experiments exploit weak, transient binding of a ligand to a target protein. The presence of excess ligand relative to protein in the sample maintains a steady state population of bound ligands. The fast koff rate enables detection of the ligands free in solution while they still retain the NMR measurable properties of the protein to which they were bound. Specifically both the NOE and transverse relaxation rate, R2, are highly dependent upon a molecule’s rotational correlation time, τC. Thus ligands that have associated with large protein molecules will exhibit much shorter T2 values relative to those that did not interact, Figure 1. For example in the case of a 300 Da fragment (0.2 nsec τC) bound to a 30 kDa protein (20nsec τC), the NOE (cross relaxation) and R2 (dipole-dipole) transverse relaxation rate of bound ligand.