Pneumology
Lung Imaging Helps to Understand Respiratory Diseases
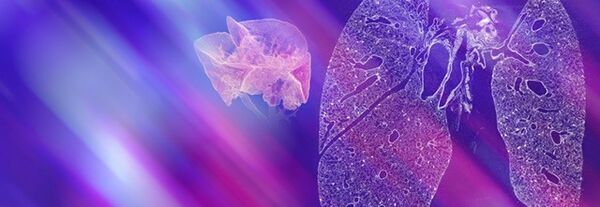
Pneumology is the prevention, diagnosis, and treatment of disorders affecting the respiratory system. In addition to respiratory disorders, such as asthma and chronic obstructive pulmonary disorder, there is a range of other chronic conditions that can affect the lungs, e.g., cardiovascular disease, cancer. Lung imaging approaches have provided health professionals with a better understanding of how the lung is affected by a range of disorders so treatments can be optimized.
Imaging methods have helped researchers determine the precise nature of the underlying causes of many lung disorders, which has significantly improved pneumology diagnoses. Preclinical small animal imaging, in particular, has provided valuable insights into the mechanisms of lung disease and the effects of treatment.
Such research using computed tomography, magnetic resonance imaging, and positron emission tomography has identified key features for confirming the diagnosis of lung disorders, for example, pneumonia, pulmonary embolism or pulmonary hypertension, early detection of lung tumors and staging bronchial carcinoma.
Bruker's extensive portfolio of molecular imaging technologies includes systems that facilitate the integration of pulmonary PET with MR, and the microCT scanner, which can be used to study live animals.
Support
Service and Life Cycle Support
Bruker’s commitment to provide customers with unparalleled help throughout the buying cycle, from initial inquiry to evaluation, installation, and the lifetime of the instrument is now characterized by the LabScape service concept.
LabScape Maintenance Agreements, On-Site On-Demand and Enhance Your Lab are designed to offer a new approach to maintenance and service for the modern laboratory


