Tech Note: Integration of Total Internal Reflection (TIRF) Microscopy with the NanoTracker 2
A thorough understanding of the dynamic processes in biological systems also requires highly specialized visualization techniques that yield information about the local distribution of cellular and molecular components contributing to or influenced by the generation of forces on the smallest scales.
In this technical note, we focus on the parallel use of TIRF and optical tweezers. Optical tweezers provide unprecedented accuracy in the measurement of single molecule forces. In combination with commercial or custom built TIRF illumination setups, Bruker's NanoTracker 2 system can deliver information on both the forces and the local dynamics of small numbers of molecules.
Readers can expect to learn about:
- The principle of Total Internal Reflection (TIRF) Microscopy;
- The full compatibility of the NanoTracker Optical Tweezers System with this technology; and
- The use of this technical combination to measure forces in the piconewton range and obtain insights into dynamic processes of molecules and cells.
KEYWORDS: Optical Tweezers; NanoTracker; TIRF; Optical Imaging; Optical Integration; Molecular Biology
Introduction
The measurement of molecular forces has gained increasing interest in biological and bio-chemical research since numerous processes were found to be regulated by local forces or highly localized changes in the mechanical properties of biological systems. Optical tweezers (OT) employ a tightly focused laser to generate a local force field that can hold ("trap") and manipulate microscopic objects, e.g. micrometer sized beads or cellular components. In addition, the forces acting on the object can be measured with high accuracy and temporal resolution. This renders OT a versatile tool for biophysical measurements whenever extremely small forces (0.1. .500 pN) are to be determined. An introduction to calibrated force measurements with OT can be found in our application note Quantitative force measurements with optical tweezers: The JPK NanoTracker™.
A thorough understanding of dynamic processes in biological systems also requires highly specialized visualization techniques that yield information about the local distribution of cellular and molecular components contributing to or influenced by the generation of forces on the smallest scales. In this technical note, we will focus on the parallel use of Total Internal Reflection Fluorescence (TIRF) microscopy and optical tweezers.
TIRF principle and optical integration
In many biological microscopy samples, the region of interest is located near the surface of a glass slide or at the bottom of a petri dish. This includes cell-surface contacts, cell membranes, adhered molecules, and other surface-bound structures. Fluorescence techniques that illuminate the bulk of the sample typically have a high background emission from fluorophores that are not located near the sample surface and thus suffer from a low signal-to-noise ratio. TIRF microscopy overcomes this problem by selectively illuminating a thin region of the sample near the glass surface. As shown in Figure 1, a laser beam is coupled into an objective with a high numerical aperture (NA 2 1.45). The NA is related to the half angle 0 of the objective's focal cone through NA = 2n sinθ where n is the refractive index of the applied immersion medium (e.g. immersion oil: n = 1.518).
The laser beam is coupled off-center into the back aperture of the objective so that it hits the sample surface at an incident angle 9. If 9in is larger than the critical angle 9crit of the glass-fluid interface at the sample surface, the beam is totally reflected, i.e. it does not directly propagate into the sample. 9crit depends on the refractive indices ng and of the glass slide and the sample, respectively: sin(θ₍crit₎) = nₛ/ng ; ng > nₛ
Near the illuminated area of the glass-sample interface, an evanescent wave forms. Its intensity inside the sample (cell, aqueous solution) decays exponentially with the distance from the surface.
The penetration depth dp of the wave depends on 9m and the wavelength of the laser light. It typically ranges from 60...200 nm which allows the selective excitation of fluorophores near the sample surface (see Figure 2). Large angles 9in result in smaller penetration depths and thus increase the localization of fluorescence excitation. The emission signal of the fluorophores is collected by the same objective and analyzed using a high sensitivity EMCCD or sCMOS camera, or similar devices.
Commercial TIRF microscopy setups like Nikon's TlTIRF illuminator couple different laser wavelengths into an inverted microscope's light path via the fluorescence illumination back port and feature motorized laser positioning and switching.
The unique design of JPK's NanoTracker™ optical tweezers setup natively supports the parallel usage of optical traps and the fluorescence port of the microscope (Figure 3). Thus, both techniques can be conveniently combined to visualize the dynamics of fluorescently labeled, surface-bound samples at high signal to noise ratios while simultaneously exerting and measuring welldefined forces.
Force measurements
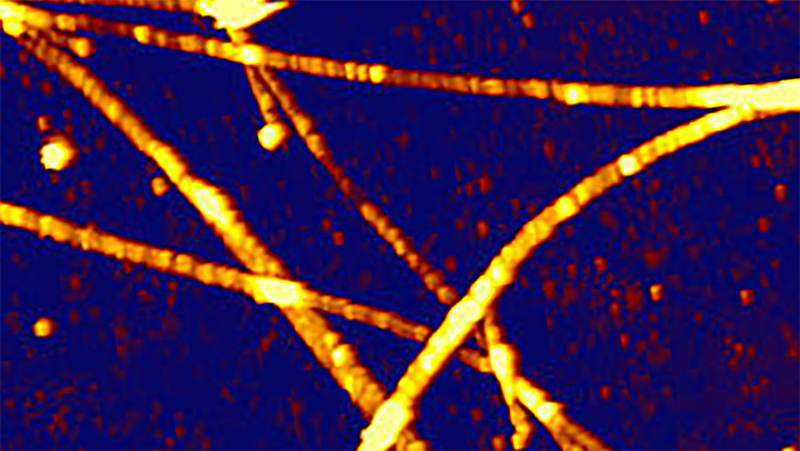
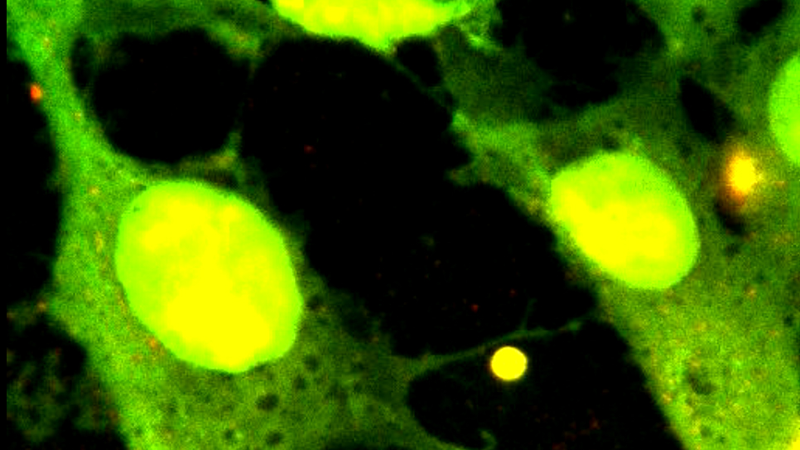
In their 2015 publication, Lansky et al. describe a combination of the NanoTracker™ system with TIRF illumination they used to investigate the force generation of microtubule cross-linking proteins[1]. A silica particle was attached to a microtubule and used as a force sensor. A second, surface-immobilized microtubule was connected via the cross-linker molecule of interest (see Figure 4). Precise sample stage movements built up mechanical stress on the cross-linker connection. The resulting force was measured and related to the local linker concentration. For this type of experiment, selective illumination is of particular advantage, since unbound proteins diffusing in the solution are not excited and, thus, do not deteriorate the signal to noise ratio.
With their OT-TIRF setup, the researchers were able to measure entropic forces in the few piconewton range, while simultaneously performing quantitative fluorescence imaging. The unique combination of mechanical and fluorescence-based quantitative measurements gives insight into the dynamic processes underlying important cellular processes.
Conclusion
Optical tweezers provide unprecedented accuracy in the measurement of single molecule forces. In combination with commercial (or custom built) TIRF illumination setups, JPK's NanoTracker™ system can deliver information on both the forces and the local dynamics of small numbers of molecules.
References
- Z. Lansky, M. Braun, A. Lüdecke, M. Schlierf, P. R. ten Wolde, M. E. Janson, and S. Diez, "Diffusible Crosslinkers Generate Directed Forces in Microtubule Networks," Cell, vol. 160, no. 6, pp. 1159-1168, Mar. 2015.