
Structure Determination
Proteins and their complexes are involved in almost all cellular processes, including metabolism, cell signaling and differentiation, and reproduction. Understanding these processes at the molecular level requires a detailed understanding the structures of the proteins themselves.
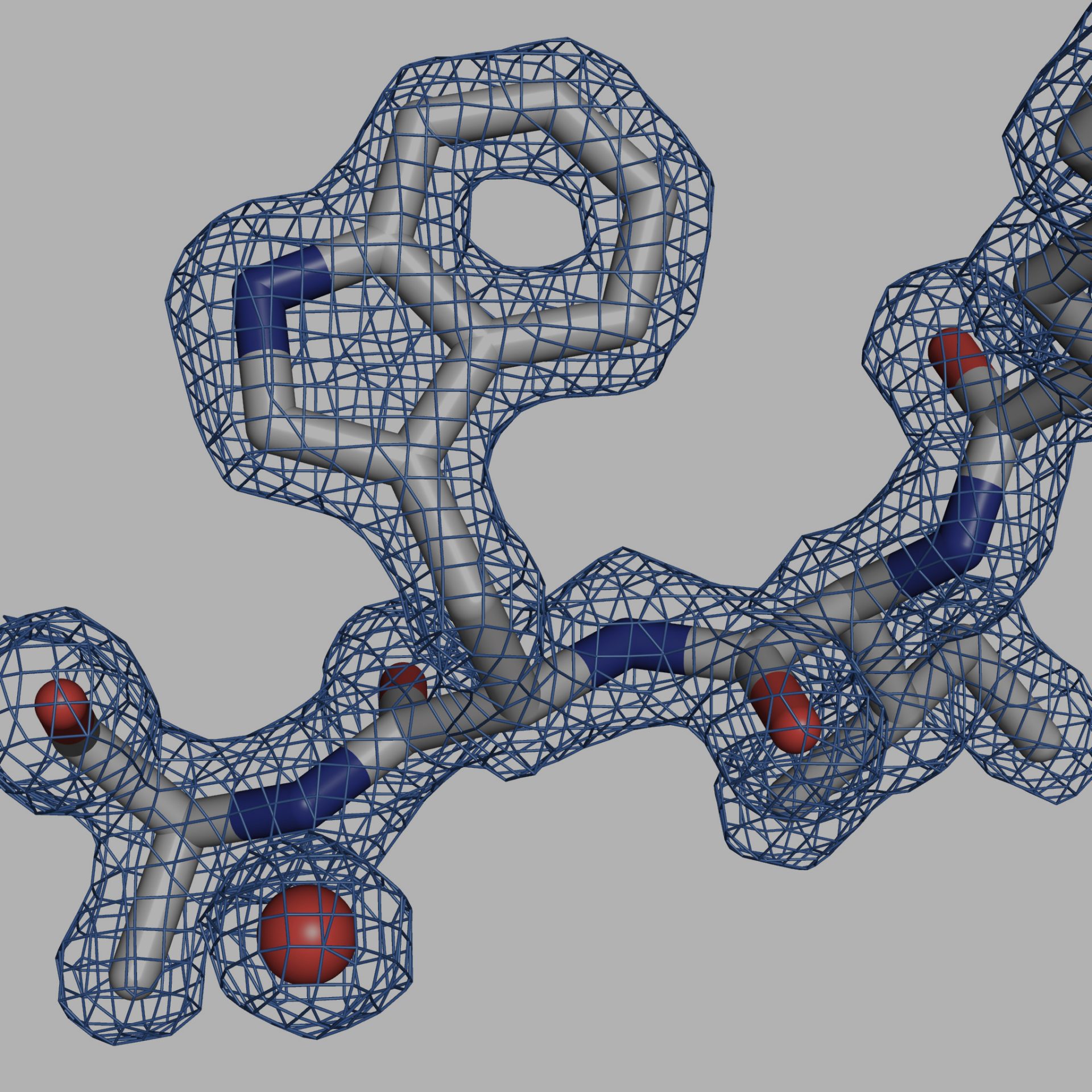
Single crystal X-ray diffraction (SC-XRD) remains the most productive technique for determining the structures of proteins and their complexes. The determination of protein structures is a labor-intensive process, and so development of home-lab SC-XRD instruments that accelerate the production and characterization of diffraction quality crystals is key to successful research. Several 100 KDa
In situ crystallography enables the analysis of crystals
directly in the lab and has such has seen a rise in use due to the
ability to optimize crystal growth conditions more quickly.
NMR is a
technique used in all Structural Biology departments to provide a
dynamic complement to the structures determined by SC-XRD.
Ground-breaking
crystallography has always been driven by the development of improved
tools. This is evident at synchrotron facilities where current beamline
upgrades are seeing the introduction of microfocus beams, faster mixed
mode photon-counting detectors, data-processing pipelines, and
multi-axis goniometers.
Bringing these possibilities to the home lab
has been Bruker’s driving vision for the D8 QUEST and D8 VENTURE, our
new D8 Structural Biology Solutions.
Why bioNMR? New series on how NMR can be used for biology research.

