NMRtist
Highlights
New Opportunities for NMR Protein Functional Analysis
Biomolecular nuclear magnetic resonance (NMR), in combination with other structural biology tools like crystallography, Cryo-EM and AlphaFold, is crucial in elucidating and understanding protein dynamics, function, structural rearrangements and ultimately disease pathways.
Automation of NMR Protein Functional Analysis Utilizing Artificial Intelligence (AI)
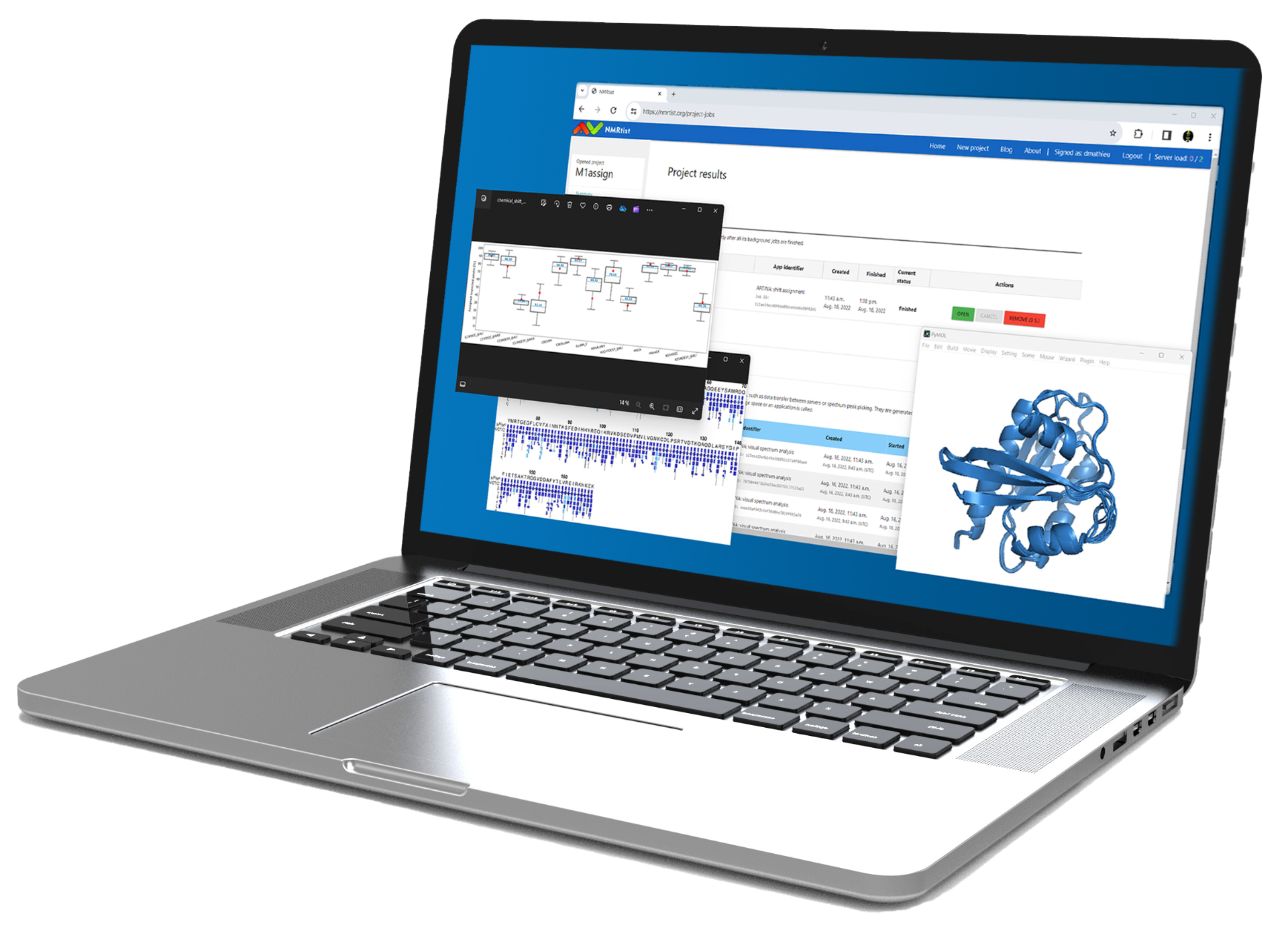
Advances in NMR technology and computational methods have significantly improved the speed and accuracy of spectral assignments, cementing biomolecular NMR’s role as an indispensable tool in modern functional structural biology. NMRtist1 is a cloud-based, AI-supported software platform developed by ETH Zurich in collaboration with Bruker to enable secure and user-friendly access to protein NMR spectra analysis. Utilizing the advanced ARTINA2,3 algorithm, NMRtist automates the lengthy and manual process of protein spectra analysis, including peak picking and assignment, reducing the timeline from weeks to just a few hours. This game-changing software provides unprecedented efficiency and reliable results with improved accuracy, making biomolecular NMR a more competitive tool for researchers in the field of structural biology.
Features & Benefits
NMRtist at a Glance:
- Cloud based, AI supported software platform
- High performance, secure cloud architecture
- Upcoming integration of automated assignments into other workflows
- Fully automated peak picking of multi-dimensional NMR spectra
- Performs resonance assignment in no time
- Fully automated structure determination
How to Get NMRtist
Getting started with NMRtist is simple and straightforward. Follow these steps to begin your journey with our cutting-edge platform to streamline protein NMR spectra analysis:
- Create an Account: Click the "Get Started with NMRtist" button below to sign up for an account and enjoy free usage of the platform for a limited time.
- License Purchase: Once the free trial is over, you can purchase a license directly on the platform to continue using NMRtist and unlock its full potential.
Cloud Computation and AI Taking the Tedious Tasks off Your Hands
The ARTINA algorithm consists of the three individual steps which can be executed separately or in consecutive order.
Peak Picking
The initial step in analyzing NMR spectra is typically peak picking. In biomolecular NMR, these spectra often have three or more dimensions, making manual peak picking or even manual inspection of peak lists an extremely time-consuming process. To address this challenge, NMRtist employs a neural network trained on a meticulously curated set of datasets, primarily comprising three- and four-dimensional protein spectra. This approach significantly contributes to the overall time savings achievable with the NMRtist platform.
Resonance Assignment
The second step is resonance assignment. What usually is a days- or weeks-long undertaking can be automated and executed in a matter of minutes or hours. The ARTINA shift assignment application first uses a deep convolutional neural network to detect positions of signals in the selected NMR spectra (see ARTINA peak picking application above). Afterwards, the detected signals undergo FLYA4 automated chemical shift assignment. The method returns protein chemical, together with assigned peak lists for the individual NMR spectra.
Backbone assignment overview including confidence levels for a 19.5 kDa protein
3D Structure Calculation
The third step is 3D structure calculation. When proteins or other biomolecules cannot be crystallized, or when AI-based predictions need verification or refinement, NMR-based structure determination becomes essential. However, this process is traditionally time-consuming and often requires an expert familiar with specialized software packages. The ARTINA structure determination application simplifies this by implementing end-to-end protein structure solving. Given a set of spectra and a protein sequence as input, the application generates the aforementioned peak lists and assignments, followed by a fully automated structure calculation using CYANA5.
Other Applications
Protein Dynamics
Easy access to assignments via the NMRtist platform benefits a wide range of applications, with the determination of backbone dynamics being just one example. A minimal set of experiments was utilized to obtain backbone amide assignments. By combining rapid pulsing and sparse sampling techniques, the total measurement time for a small protein was reduced to just under 2 hours.
NMRtist successfully achieved a complete backbone assignment, which was then used alongside relaxation measurements to assess the rigidity of the protein backbone using DynamicsCenter™. The integration of bioTop for setup and acquisition, NMRtist for obtaining assignments, and DynamicsCenter for advanced data analysis creates an almost fully automated, straightforward method.
Prof. Dr. Roland Riek
Professor of Physical Chemistry and head of the Bio-NMR group at the Department of Chemistry and Applied Biosciences at the Swiss Federal Institute of Technology (ETH) Zürich
"The NMRtist platform is a significant step forward for the field of structural biology. The ability to automate the labor-intensive process of analyzing higher dimensionality spectra will provide much easier access to spectral assignment as well as 3D protein structures by NMR, which has been a time-consuming task for a trained expert in the field of biomolecular NMR."
Next Steps
Working together with the developers at the ETH in Zurich, Bruker will provide the users with a high performance, secure platform to run ARTINA jobs. Security will be provided using state of the art cloud infrastructure as well as regular security audits by 3rd parties.
In addition, Bruker will provide and interface from within TopSpin and bioTop to integrate the NMRTist platform seamlessly into other workflows, for example using automated assignment results to determine protein dynamics.
Requirements
- NMRtist works with various formats of processed NMR data incl. data sets in TopSpin format
- Minimum combination of required data sets largely depends on molecular size and complexity
Webinars
More Information
Related Products
References
- Klukowski, P., Riek, R. & Güntert, P. NMRtist: an online platform for automated biomolecular NMR spectra analysis, Bioinformatics, 2023.
- Klukowski, P., Riek, R. & Güntert, P. Rapid protein assignments and structures from raw NMR spectra with the deep learning technique ARTINA (2022). Nature Communications 13, 6151.
- Klukowski, P., Riek, R. & Güntert, P. Time-optimized protein NMR assignment with an integrative deep learning approach using AlphaFold and chemical shift prediction, Science Advances, 2023.
- Schmidt, E., & Güntert, P. (2012). A new algorithm for reliable and general NMR resonance assignment. Journal of the American Chemical Society, 134, 12817-12829.
- Güntert, P. & Buchner, L. (2015). Combined automated NOE assignment and structure calculation with CYANA. J. Biomol. NMR 62, 453-471.