
Dissolution
Approximately 80% of all pharmaceutical products are delivered in tablet form. Tablet formulations are designed to deliver the Active Pharmaceutical Ingredient (API) to the body in a predictable and consistent fashion depending on the release characteristics required so that the intended performance is delivered. There is a necessity to establish the dissolution performance of the tablet as this is a critical quality attribute (CQA).
A range of different tablet formulation types exist and, for example, the properties of the enteric coating as well as the design of the tablet itself, which may be constrained within a matrix construct or as an osmotic pump, will affect the release characteristics of the API. As a consequence, improved methods to visualise and measure the properties of enteric coating and API hydration are important parameters to quantify improving the degree of understanding of the tablet design itself and provide important intellectual property to protect generic manufacture of the tablet formulations.
During the manufacturing process, slight deviations in humidity and temperature can detrimentally impact the properties of the formulation which can result in accelerated, delayed or inconsistent release of the API depending on the location within the gastrointestinal tract.
Bruker offers a range of technologies that can be used to study dissolution and the physical structure of tablets.
MRI, using Bruker BioSpec MRI can be used to longitudinally measure water ingress (diffusion), swelling, cracking of the enteric coat and temporal release characteristics of the API under differing pH conditions. All these characteristics can be measured with absolute quantification from 2 or 3D images acquired. In addition, as well as tablet dissolution characteristics NMR can be used to directly measure the release of the API and /or excipients using on-line monitoring with the insightMR to correlate with the images from the MRI. Impurities and macroscopic assessments are another important consideration and using Bruker’s SkyScanner micro CT sub micron evaluations can be made on the whole tablet.
Videos
These videos were recorded on NMR spectrometers incorporating NMR Microscopy (Microimaging) accessories. Standard bore or wide bore magnets were used, operating at 7 T or 11,7 T. The methods are 2D or 3D spatially resolved MRI sequences with different repetition times, adapted to the dissolution time of the tablets and to the requested image quality.
Microimaging of Tablets:
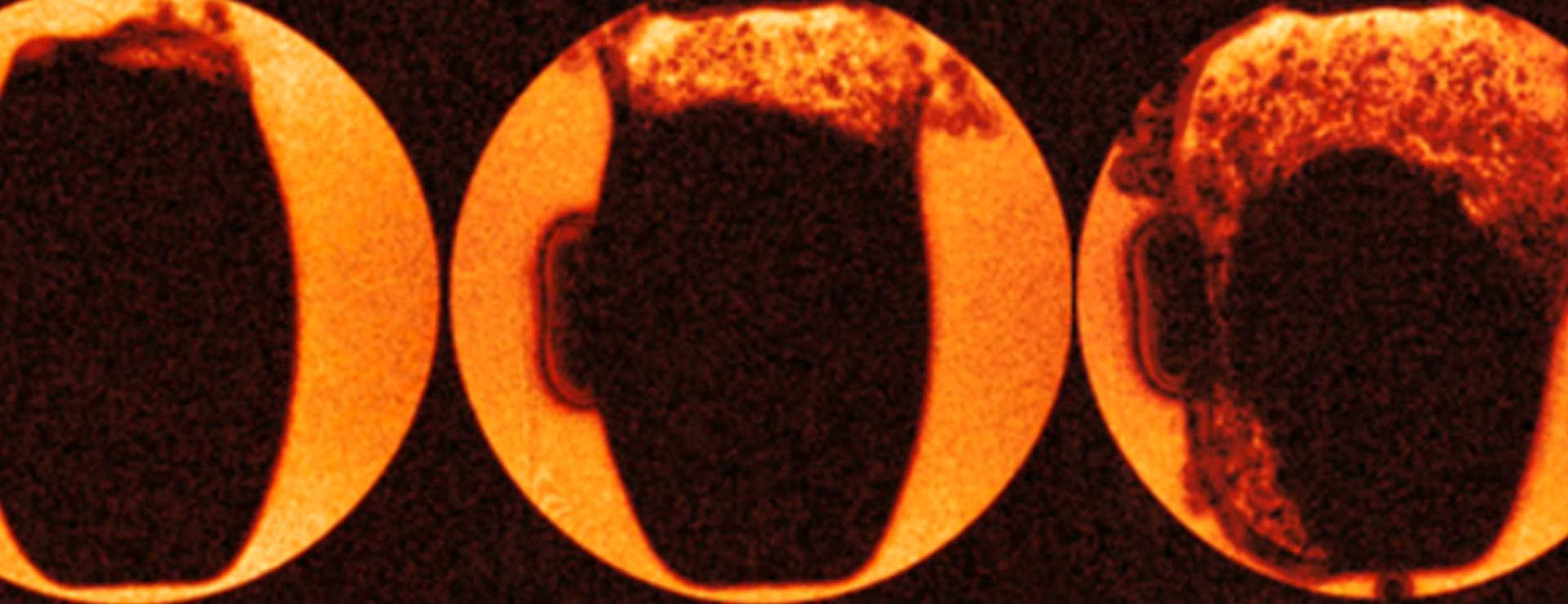
Behaviour of a tablet as water diffuses through the outer shell. The rapid “explosion” of the tablet starts in the top right-hand corner of this image and indicates that there might be a fault in the coating in that location. The “artefact” to the left was caused by paramagnetic components in a label stamped into the tablet and located in that area.
Immediate release tablet: two tablets have been infused with Silicone oil to visualize the structure of the tablets – the upper tablet has multiple bright spots which indicate that there are voids within the tablet and therefore there has been a manufacturing fault. The different brightness of the two tablets shows the different tablet densities.
Here, a tablet has been infused with silicone oil, and the soft core and hard coat are clearly visible. Also, high density structures at the surface close to the central (horizontal plane) are visible. Surface distortions were observed, where these structures reach the coat
LabScape
Service & Life Cycle Support for Magnetic Resonance and Preclinical Imaging
Bruker’s commitment to provide customers with unparalleled help throughout the buying cycle, from initial inquiry to evaluation, installation, and the lifetime of the instrument is now characterized by the LabScape service concept.
LabScape Maintenance Agreements, On-Site On-Demand and Enhance Your Lab are designed to offer a new approach to maintenance and service for the modern laboratory