
Receptor-Ligand Interactions
Quantifying Binding-Affinity and Cellular Responses
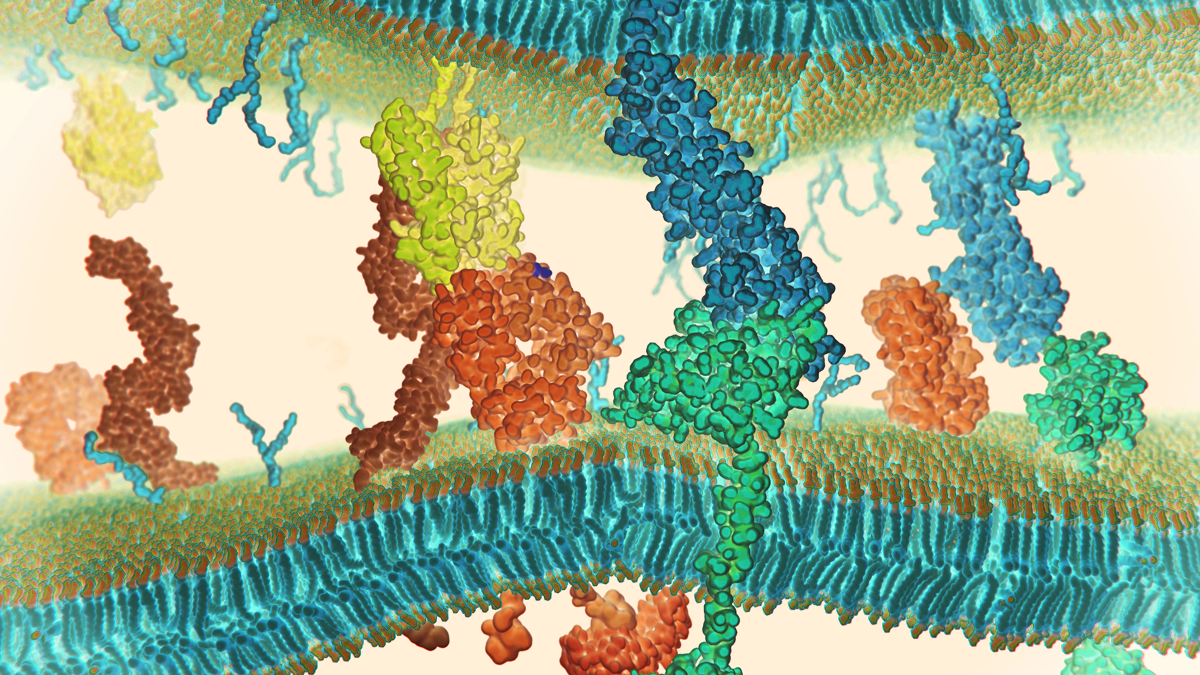
Proteins, drugs or pathogens binding to cell-surface receptors can trigger cellular response at the molecular and macroscopic level. Receptor-ligand interactions are a major class of protein-protein interactions and play an important role in many biological processes such as metabolism, neurotransmission and cellular signal transduction pathways.
When a ligand binds to a protein, it undergoes a conformational change which in turn leads to a physiological response. The time a ligand spends attached to a receptor or specific protein is a function of the affinity between the ligand and the protein. The higher the affinity, the stronger the ligand will bind to the protein, and the longer the functional modification with last. The lower the affinity, the less like it is to bond at all or the faster the release from the receptor will occur.
Quantifying the binding affinity of a ligand is therefore essential, as it determines the potency of the ligand. Nanoanalytical tools like atomic force microscopy (AFM) and optical tweezers have increasingly been used to analyze "receptor" function, whereby the adhesion forces between ligands and receptors in cells can be accurately measured and mapped, and downstream cellular responses analyzed.