
Protein Crystallography & Structure-Based Drug Design
Single Crystal X-Ray Diffraction for Protein Analysis, Drug Discovery & Development
Bruker offers a comprehensive portfolio of single-crystal X-ray diffraction (SC-XRD) systems tailored for pharmaceutical research and development - ideal for everything from compact academic labs to high-throughput industrial facilities.
From solving the structure of proteins and other macromolecules to researching promising small molecules, determining the chirality of an API, or performing solid form analysis, Bruker has the solution for you.
Bruker SC-XRD systems are designed to support and accelerate research in the life sciences and pharmaceutical industry.
Our SC-XRD portfolio includes a range of systems for structural biologists and chemists, ranging from compact systems for high-quality structure determination to high-throughput platforms for demanding projects.
Find out more about the application of our SC-XRD systems in protein crystallography, small molecule analysis and solid form development here.
Protein Crystallography
Protein crystallography is a method used to determine the three-dimensional structures of proteins at atomic resolution. The technique involves diffracting X-rays through protein crystals followed by analysis of the diffraction patterns.
The data collected via protein crystallography provides detailed insights into the shape, folding, and functional sites of biological macromolecules, helping to understand their biochemical roles and mechanisms of action.
Researchers use protein crystallography to validate drug targets by visualizing active sites, binding pockets, and conformational states. it confirms a target’s role in disease and its potential for therapeutic modulation. This structural insight confirms a target’s role in disease and ensures that only the most promising targets advance in discovery.
This information is vital for structure-based drug design (SBDD), enabling the precise targeting of active sites by molecules with optimized binding affinity and specificity.
Advances in single crystal X-ray diffraction instrumentation have made it possible for laboratories to now obtain high-quality structural data in-house, further accelerating structural biology research and pharmaceutical development.
Solve Complex Protein Structures In-House
Protein crystallography is a critical tool in pharmaceutical research. However, researchers often face challenges such as small, weakly diffracting crystals and limited access to synchrotron facilities, which can delay project timelines.
The D8 VENTURE METALJET is designed to address these challenges with high-intensity, synchrotron-like X-ray beams and advanced detectors, enabling the collection of high-quality data, even from challenging samples, in-house. Automated features support unattended data collection and fast processing, helping to increase throughput and reduce bottlenecks.
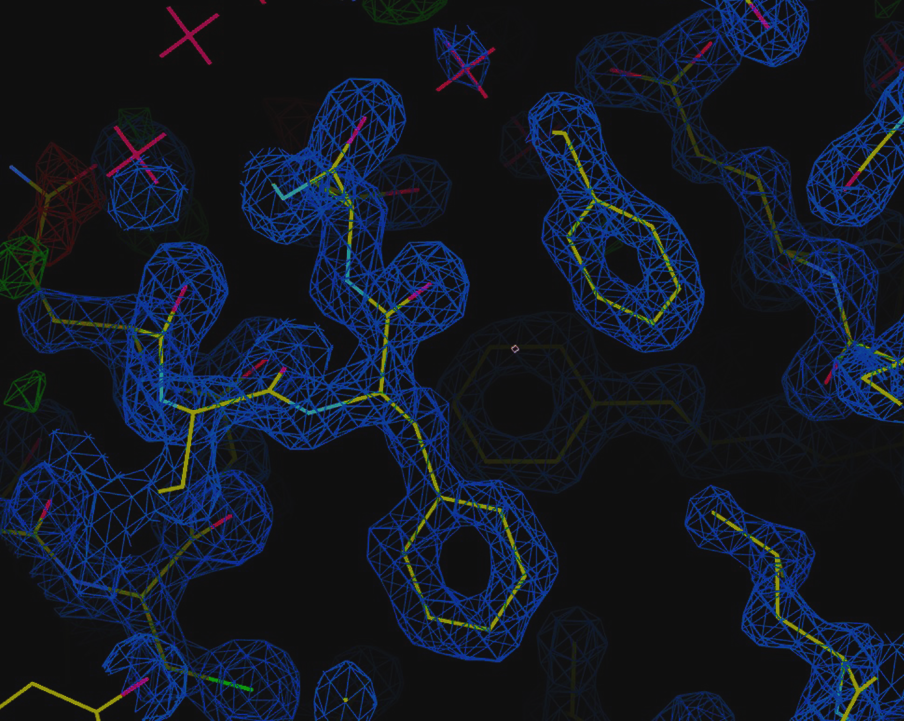
Case Study: Solving the Structure of a MTase Complex
Figure 2 shows the crystal structure of a protein complex - nsp16 (MTase), and nsp10 (a cofactor) - with sinefungin (an inhibitor) bound to its active site.
MTase is a coronavirus enzyme that, when complexed with the nsp10 co-factor, plays a crucial role in RNA cap formation, making it essential for viral RNA stability. As such, the MTase complex is a promising target for antiretroviral therapy.
Using the D8 VENTURE METALJET researchers were able to model the 3D molecular structure of the complex, identifying the binding site of the inhibitor, sinefungin, which is located next to the RNA binding pocket.
Krafcikova et al., Nature Communications, Structural analysis of the SARS-CoV-2 methyltransferase complex involved in RNA cap creation bound to sinefungin.
Optimize Crystallization Conditions to Solve Target Structures Faster
Developing well-diffracting crystals for pharmacological targets is a key step in structure-based drug design (SBDD), but it can be complex and time-consuming.
When researchers are unable to produce suitable crystals, projects can be delayed or even halted. This can be especially costly for SBDD projects focused on high-value targets like epigenetics or GPCRs.
Having access to in-house SC-XRD systems such as the D8 VENTURE allows researchers to explore crystallization conditions and optimize crystal quality. This flexibility supports faster decision-making and improves the chances of successful outcomes.
Automated data collection further enhances efficiency. The D8 VENTURE, equipped with SCOUT automated sample handling, can deliver synchrotron-quality datasets in hours or even minutes. This capability helps clear backlogs and fill gaps in beamtime, supporting continuous progress in pharmaceutical research.
Case Study: Solving the Structure of BRD4 with Data Collected in 15 Minutes
BRD4 is a protein that plays a key role in regulating gene expression and is considered an important target in SBDD, particularly for cancer and inflammatory diseases.
Using the D8 VENTURE researchers were able to solve the BRD4 structure (figure 3) at 1.7 Å resolution from a complete dataset collected in just 15 minutes, demonstrating the efficiency and precision of in-house SC-XRD for drug discovery.
Absolute Structure Determination of Small Molecules
Visualizing the three-dimensional atomic structure of an active pharmaceutical ingredient (API) helps to develop understanding of its chemistry and intermolecular interactions, with this information guiding drug development.
The structural information provided by single crystal x-ray diffraction is crucial for optimizing molecular properties and advancing promising compounds through the development pipeline. Moreover, SC-XRD enables researchers to examine features such as hydrogen bonding networks, conformational flexibility, and intermolecular interactions that can influence a compound’s pharmacokinetic and pharmacodynamic profiles.
Determine the Chirality of Compounds with SC-XRD
One of the key advantages of SC-XRD is its ability to determine the absolute configuration of chiral molecules at atomic resolution.
For APIs containing chiral centers, knowing the exact stereochemistry is essential for guiding synthetic strategies and meeting regulatory requirements. Consequently, SC-XRD is the only experimental technique that can unambiguously confirm these configurations, ensuring the integrity and efficacy of new drug candidates. This reliability enhances confidence in the development process and facilitates efficient progression from lead discovery to regulatory submission.
Rapid and Reliable Structural Determination of APIs
The D8 QUEST is designed to deliver rapid and reliable results. Equipped with high-intensity microfocus X-ray sources and advanced photon-counting detectors, these instruments can solve atomic resolution structures from even the smallest crystals in just minutes. This speed and accuracy allow researchers to routinely determine absolute structures and analyze complex APIs without lengthy delays.
Solid Form Analysis
Solid form analysis allows researchers to characterize and understand the detailed atomic arrangement of molecules within solid-state materials.
SC-XRD offers unparalleled resolution of both molecular conformation and the precise nature of intermolecular interactions present in different crystalline forms. Consequently, researchers can identify polymorphic phases, cocrystals, solvates, and hydrates - each of which may profoundly influence the stability, solubility and bioavailability of an API.
The atomic-scale structural information provided by SC-XRD provides deeper insights into polymorphism, enabling the production of pharmaceutical compounds of greater stability, tailored for manufacturability and improved patient outcomes.
Fast and Accurate Advanced Solid Form Analysis
Bruker’s D8 QUEST and D8 VENTURE systems allow researchers to determine structures directly from tiny crystallites selected from bulk powder, eliminating the need for time-consuming recrystallization and ensuring the atomic structure matches the bulk material. These systems also support non-ambient analysis, enabling studies of temperature, pressure, and hydration effects on solid forms.
Bruker’s solutions deliver atomic resolution structures from single crystallites of less than ten microns, making solid form analysis simple and reliable. Modular configurations let laboratories select the right source and detector for their needs, supporting advanced studies like micro powder diffraction and PDF analysis.
With Bruker’s SC-XRD systems, pharmaceutical labs can quickly and accurately characterize new solid forms, understand polymorphism, and engineer more stable crystal forms. This capability helps overcome bottlenecks in solid form optimization and supports the development of APIs with suitable stability, manufacturability, and metabolic behavior.
Find the Best SC-XRD Solution for Your Life Science and Pharmaceutical Research
Bruker offers a range of SC-XRD systems designed to meet different research needs and budgets in life science research and pharmaceutical industries. Each instrument can be customized to a configuration that best fits your analytical requirements and operational goals.
Download our Crystallography Solutions Brochure to see a detailed system comparison of features such as source intensity, detector size, automation options, and throughput capabilities to help you identify which Bruker SC-XRD solution best fits your research needs.
If you need further guidance, you can also contact one of our experts to help determine the ideal system for your specific applications.