Drug Manufacturing
Valuable solutions for drug manufacturing
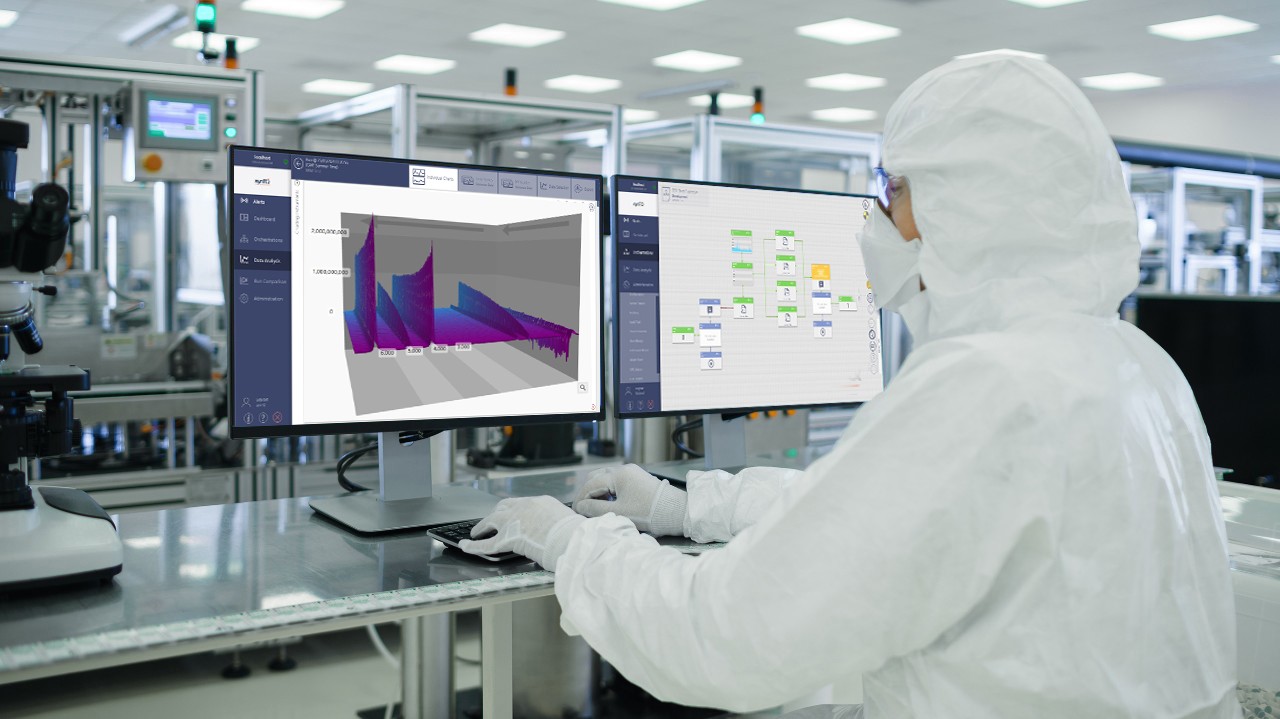
Market drivers - from payors, patients and regulators - mean that pharma and biopharma manufacturing is under constant pressure to increase efficiency, reduce cost and maintain quality. Many forward-thinking companies have invested in smarter manufacturing, putting lean, six sigma PAT and QbD principles into routine practice. Many are now considering a move to continuous manufacturing process. The goals are clear, build in regulatory compliance, ensure close monitoring of processes, de-risk the manufacturing chain and enable fast drug release. But achieving success is not just about process, robust analytical information is key.
Bruker's suite of mass spectrometers, XRD and XRF analyzers, NMR systems, FT-IR/FT-NIR and Raman instruments provide valuable solutions for both small molecule and biopharmaceutical drug manufacturers.