Antimicrobial susceptibility testing
MICRONAUT and UMIC® for high accuracy susceptibility testing
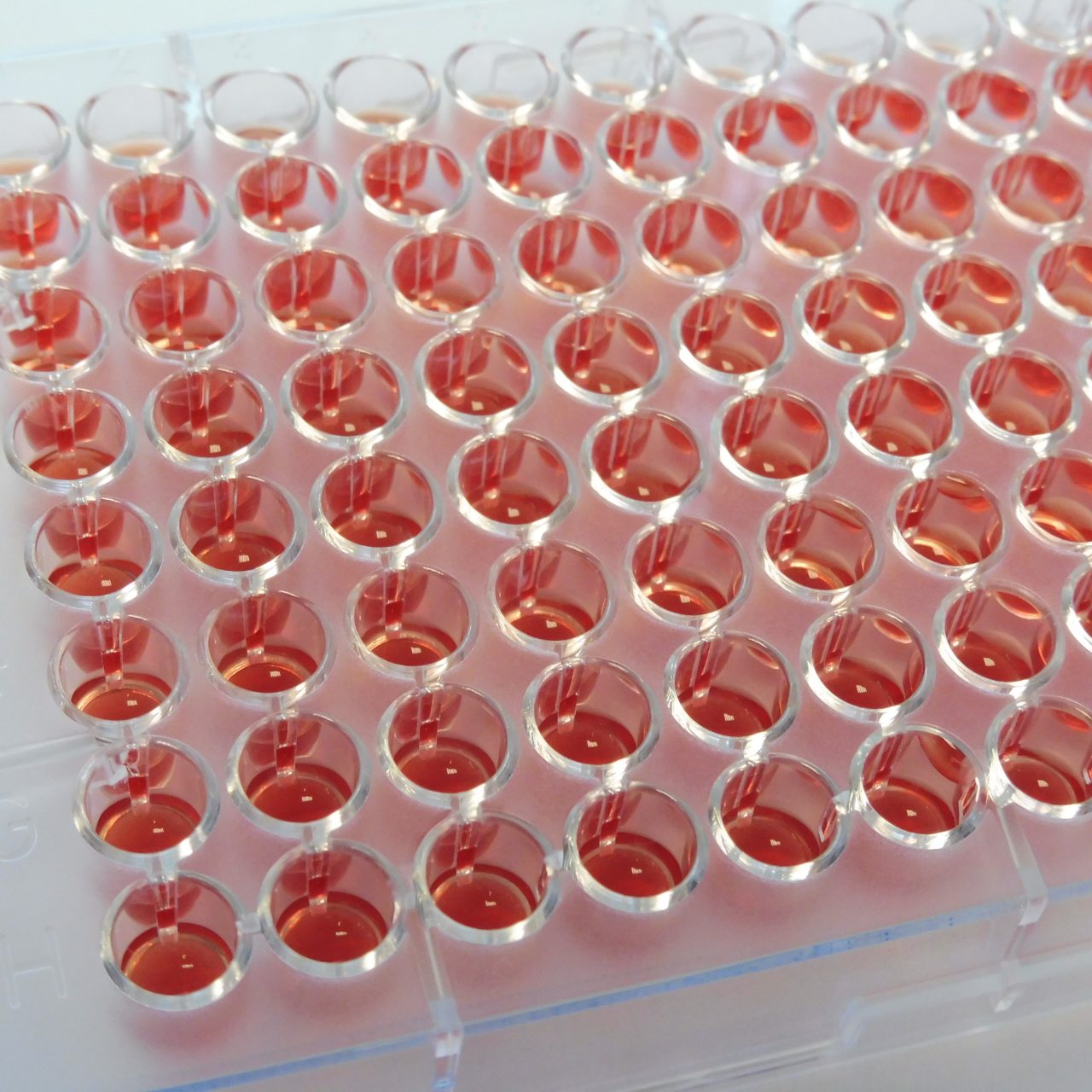
Detection of resistance mechanisms and quantification of microbial resistance by determination of true MICs is performed using the MICRONAUT and UMIC® broth microdilution (BMD) product portfolios.
Overuse and misuse of antibiotics and antifungal agents has led to the emergence of Multi-Drug Resistant microorganisms. This translates into situations where clinicians are lacking treatment options. The increasing prevalence and severity of bacterial and fungal infections in healthcare scenarios requires a standardized routine antimicrobial/antifungal susceptibility test (AST/AFST) in the microbiology lab.
While having to make the right decision with regards to patient treatment escalation or de-escalation in the context of AMR, antibiotic stewardship teams look at preserving the long-term efficacy of antibiotics and antifungal agents. For them, it is critical to obtain accurate AST/AFST results from the lab that they can trust. Clinical microbiology laboratories need an accurate and reliable MIC method as an addition to their routine AST workflow. A flexible solution providing EUCAST and/or CLSI compliance, simplicity and convenient logistics is therefore required to resolve current limitations.
MICRONAUT products are based on the worldwide accepted reference method of broth microdilution, therefore delivering MIC results of highest accuracy. A variety of MICRONAUT products is available, e.g. for reliable antifungal susceptibility testing of yeasts and cryptococci according to latest EUCAST standards (MICRONAUT-AM) and for efficient susceptibility testing of clinically relevant anaerobic bacteria (MICRONAUT-S Anaerobes MIC). Long shelf life and storage at room temperature simplify logistics and keep costs under control.
UMIC® is Bruker´s brand for high accuracy single drug MIC testing based on broth microdilution reference method. They are ready-to-use unitary strips with 12 wells each, containing dried antibiotics and prepared according to the ISO 20776-1 standard. UMIC® allow flexibility in the choice to test one or several drugs for a clinical isolate, from the same bacterial inoculum. A product range covering critically important antibiotics is available. UMIC® test kits available for: Colistin, Piperacillin-Tazobactam, Vancomycin-Teicoplanin, Daptomycin, Linezolid, Cefiderocol.
UMIC® are easy to implement in routine and can be used in combination with UMIC® Incubation boxes to preserve humid atmosphere and to allow easy recognition in the incubator. They also serve as a reading tool, with a reminder of the concentrations of the antibiotic present in the wells of the UMIC®. Long shelf life and storage at room temperature simplify logistics and keep costs under control.
Development and manufacturing of MICRONAUT and UMIC® products is done by MERLIN Gesellschaft fuer mikrobiologische Diagnostika mbH - a Bruker company since 2017. The products are used in routine laboratories in the field of human medicine as well as in veterinary medicine.