Two-photon-based Phosphorescence Lifetime Imaging (2P-PLIM) for in Vivo Oxygen Measurements at High Spatial and Temporal Resolution
Find out what oxygen imaging can reveal about brain activity and metabolism
Dr. Martin Thunemann discusses his novel approach to exploring neural activity and oxygen concentrations in anesthetized and awake rodents. Two-photon-based phosphorescence lifetime imaging (2P-PLIM) allowed measurements to be taken in the brain at a high temporal resolution. This imaging approach may also be used in areas other than neuroscience such as oncology and physiological experiments.
Webinar Abstract
Phosphorescence lifetime imaging has been gaining popularity in the two-photon microscopy world and is attributed to the development of two-photon-excitable oxygen-sensing probes [1, 2]. The lifetime of their phosphorescence is in the microsecond range and is shortened in the presence of molecular oxygen, a process called "oxygen-dependent quenching of phosphorescence". Two-photon-based phosphorescence lifetime imaging microscopy (2P-PLIM) enables the quantitative estimation of oxygen concentration as the partial pressure of oxygen (pO2) within a given tissue in vivo and at a high spatial resolution. Rapid and frequent phosphorescence lifetime acquisition cycles supported through microscope hard- and software allow users to perform functional measurements at high temporal resolutions.
We use 2P-PLIM to measure pO2 in the brain of anesthetized and awake rodents [3, 4] aiming to understand the intricate balances at play. Specifically, between neuronal activity-driven oxygen consumption and oxygen delivery from the blood, which is the underlying mechanism of human neuroimaging studies based on functional MRI [5]. However, 2P-PLIM can also be used in areas other than neurosciences, e.g., to study tumor microenvironment, inflammation, and other physiological or pathological processes in vivo.
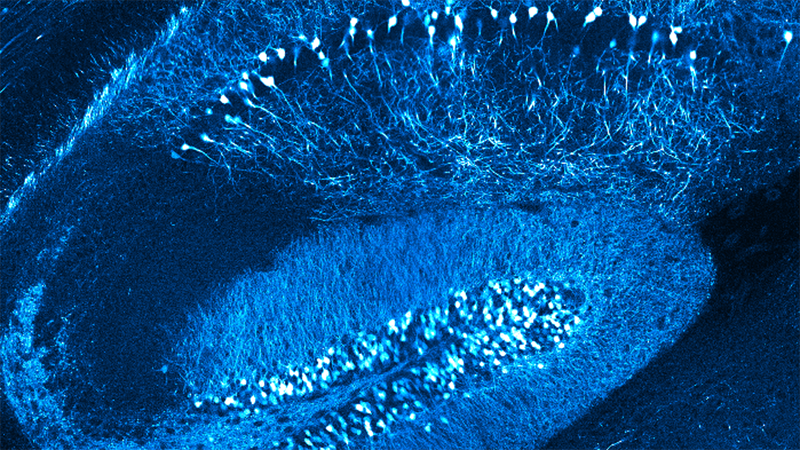
Here, we show tissue pO2 measurements with Oxyphor 2P [2] in the somatosensory cortex of awake head-fixed mice. Oxyphor 2P was microinjected through a silicone port below a cranial window. 2P-PLIM was performed with a Bruker Ultima 2-photon laser scanning microscope equipped with a GaAs photomultiplier module that was connected to a time-correlated single-photon counting card integrated into the microscope’s hard- and software. Tissue pO2 was measured in the vicinity of a "diving" arteriole which is a small blood vessel that delivers oxygen-rich blood from the brain surface to deeper cortical layers. As expected, we observed spatial pO2 gradients around the diving arteriole, from high pO2 close to the arteriole to lower pO2 further away from the arteriole [2, 3]. Currently, we use these measurements to estimate laminar profiles of cell-type-specific contributions to oxygen consumption in the cerebral cortex.
[1] Finikova, et al., ChemPhysChem 2008, 9(12):1673-79. DOI: 10.1002/cphc.200800296.
[2] Esipova, et al., Cell Metabolism, 2019, 29(3):736-744. DOI: 10.1016/j.cmet.2018.12.022.
[3] Sakadžić, et al., Nature Methods 2010, 7(9):755-759. DOI:10.1038/nmeth.1490.
[4] Sakadžić, et al., Neurophotonics 2016, 3(4). DOI: 10.1117/1.NPh.3.4.045005.
[5] Uhlirova, et al., Philosophical Transactions of the Royal Society B: Biological Sciences 2016, 371(1705):20150356. DOI: 10.1098/rstb.2015.0356.
Find out more about the technology featured in this webinar or our other solutions for Multiphoton Microscopy:
Featured Products and Technology
Speakers
Dr. Martin Thunemann
Dr. Thunemann received his doctoral degree in Biochemistry from Eberhard Karls Universität in Tübingen, Germany. During his graduate studies, Dr. Thunemann gained extensive knowledge in molecular imaging, microscopy, cardiovascular physiology, and mouse transgenesis. He joined the Neurovascular Imaging Laboratory headed by Dr. Anna Devor at UC San Diego in 2015 as postdoctoral fellow and later as assistant project scientist. He is leading several bioengineering efforts including projects based on advanced multiphoton imaging methods as well as multiplexed electrophysiological recordings in awake behaving mice. Dr. Thunemann recently joined the Department of Biomedical Engineering at Boston University as Research Assistant Professor.