A Spike-Timing Dependent Plasticity Rule for Dendritic Spines
Learn about a plasticity rule governing the location and structure of dendritic spines.
Attendees will learn how the use of multiphoton glutamate uncaging can mimic synaptic release and uncover the mechanisms of spike-timing-dependent plasticity. This approach reveals insights into the location and structural organization of dendritic spines and has useful implications for learning, memory, and cognition.
Dr. Araya's Abstract
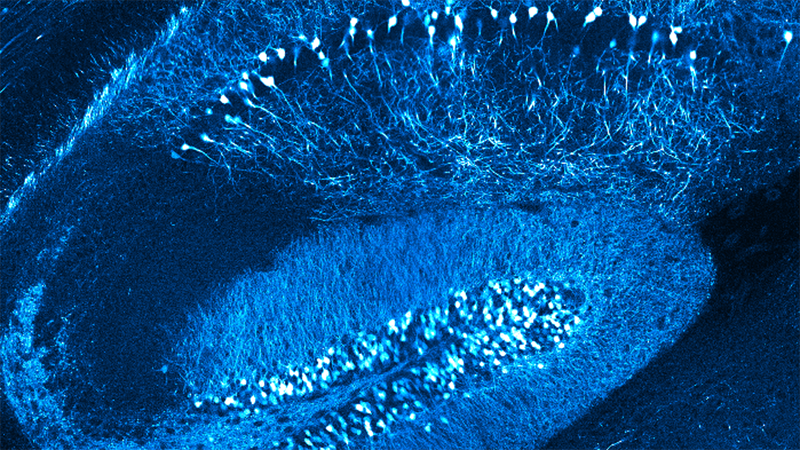
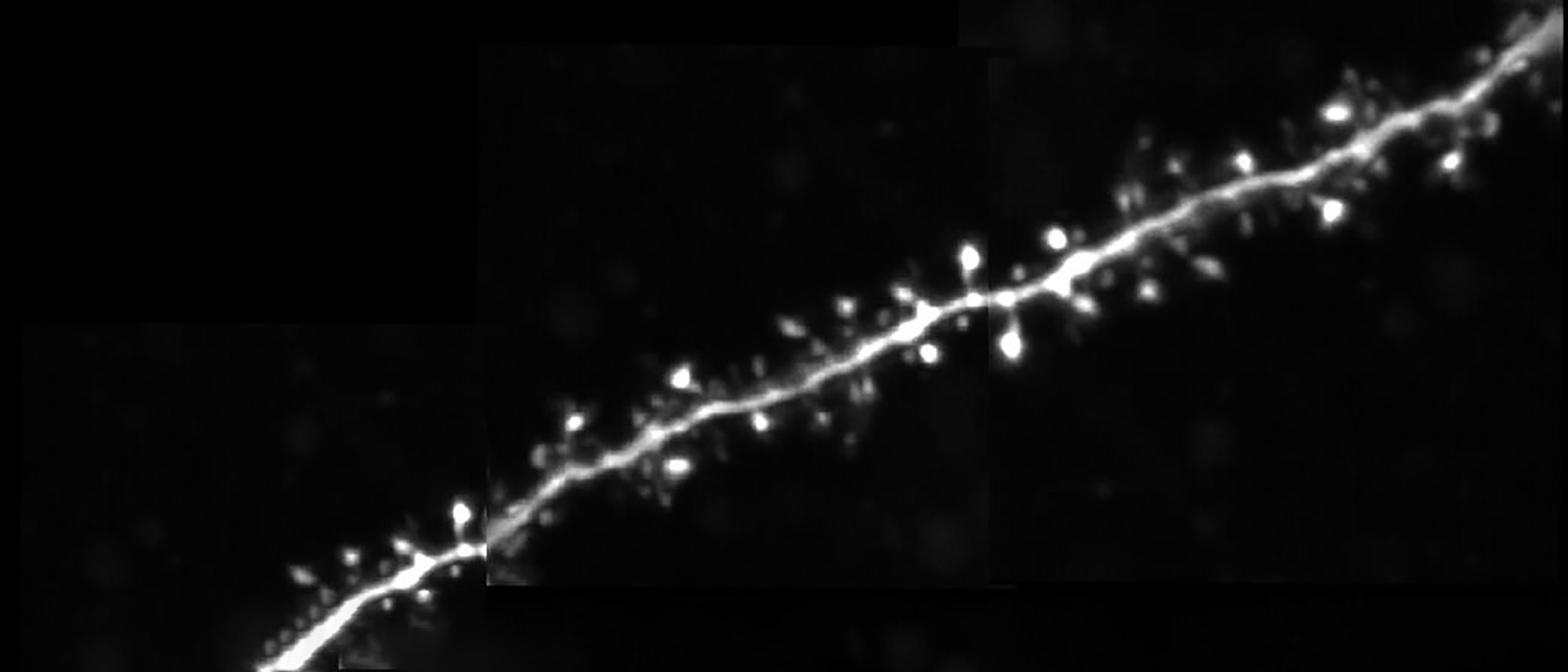
Dendritic spines, the main recipient of excitatory information in the brain, are tiny protrusions with a small head separated from the dendrite by a slender neck. Spines can undergo structural remodeling that is tightly coupled with synaptic function. They are also the preferential site for the induction of long-term potentiation (LTP) and long-term depression (LTD) thought to be the underlying mechanisms for learning and memory in the brain. A variation of LTP and LTD has been described in pyramidal neurons that involve the pairing of pre- and postsynaptic action potentials (APs), known as spike-timing-dependent plasticity (STDP). In this process, the timing between pre- and postsynaptic APs modulates synaptic strength, triggering LTP or LTD.
The sign and magnitude of the change in synaptic strength depend on the relative timing between spikes of two connected neurons (the pre- and postsynaptic neurons). The STDP learning rules have been extracted from studies using connected neuronal pairs or by using extracellular stimulating electrodes, but the precise location and structural organization of the excitatory inputs that support STDP at its minimal functional unit—the dendritic spine—are unknown.
In this talk, Dr. Araya provides evidence showing that the induction of STDP in single or distributed spines from layer 5 (L5) pyramidal neurons follows a bidirectional Hebbian STDP rule. This conclusion is explored using two-photon (2P) glutamate uncaging that mimics synaptic release. Furthermore, he shows that synaptic cooperativity, induced by the co-activation of only two clustered spines using 2P glutamate uncaging, disrupts t-LTD (<40 µm distance between spines) and extends the temporal window for the induction of t-LTP (<5 µm distance between spines). This is due to the generation of differential local N-methyl-D-aspartate (NMDA) receptor-dependent calcium signals, which leads to an STDP rule for clustered inputs only encompassing LTP.
These findings suggest that the functional specificity and structural arrangement of synaptic inputs, distributed or forming micro-clusters in the dendrites of pyramidal neurons, are fundamental for guiding the rules for sensory perception, affecting the STDP learning rule, learning and memory, and ultimately cognition.
Find out more about our other solutions for Fluorescence Microscopy or Multi-Photon Microscopy:
Featured Products and Technology
Guest Speaker
Dr. Roberto Araya
CHU Sainte-Justine Research Centre and University of Montreal
Dr. Araya completed his graduate studies with a joint thesis at the Catholic University in Chile and the University of Bonn in Germany. For his post-doctoral training, Dr. Araya joined the laboratory of Dr. Rafael Yuste at Columbia University. He was funded by a Latin American PEW fellow in biomedical sciences and later by the Howard Hughes Medical Institute. In 2011, he was hired in the Department of Neuroscience at the University of Montreal. Dr. Araya recently joined the CHU Sainte-Justine Research Centre as an Associate Professor to form part of the Child and Brain Development research axis, with the goal to further establish collaborations and pursue translational work in autism spectrum disorders. His research is well funded by the Canadian Institute of Health Research and other institutions and foundations.
Dr. Araya's research focuses on understanding how neocortical pyramidal neurons, and the circuitry they reside in, enables sensory processing. More specifically, Dr. Araya is passionate about dendritic computations, and how dendritic spines control the processing, storage, and integration of synaptic inputs. He is extending these questions to neurological disorders to uncover abnormal neuronal and circuit elements via a multifaceted approach that includes a state-of-the-art custom-built in vivo and in vitro two-photon setup with holographic stimulation capabilities and electrophysiological, structural, genetic, and molecular tools.