
Orthopedic Implants
Building bodies for longer lives
Orthopedic Implants
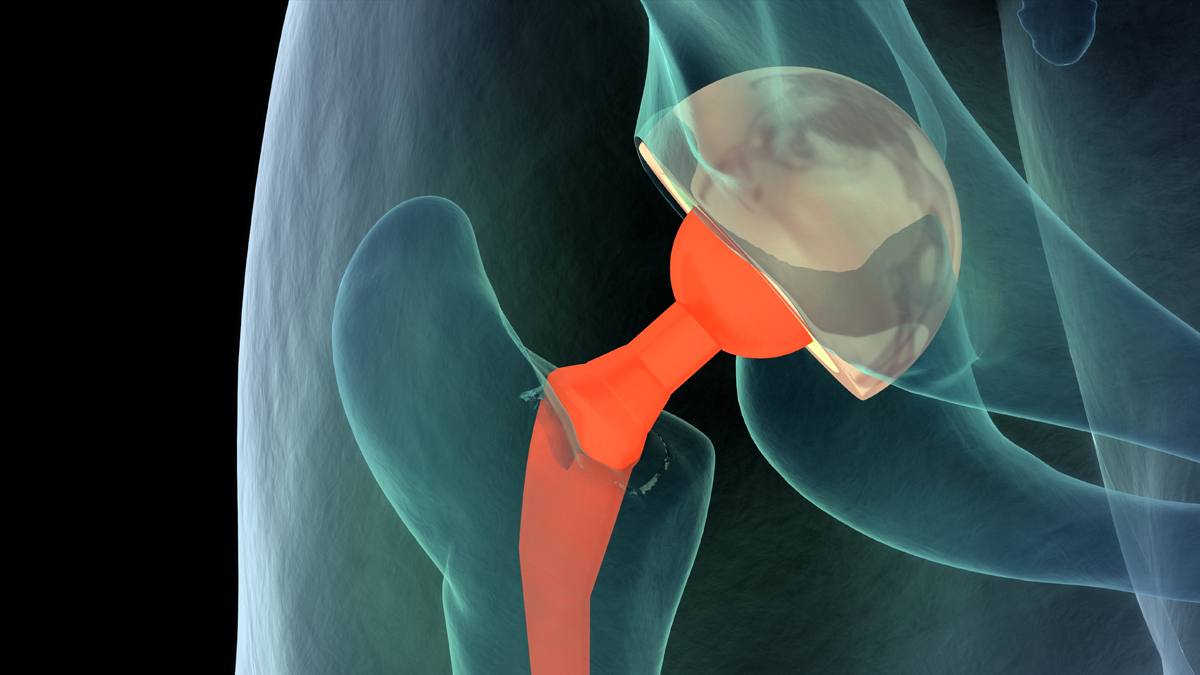
More than one million joint implants are currently produced every year, with a growth rate of nearly 9%. Lifetime and safety expectations continually increase, which place ever tighter requirements on the development and manufacturing processes. Both shape and roughness are critical parameters that must be controlled to ensure proper function and lifetime of orthopedic implants.
Surface Metrology
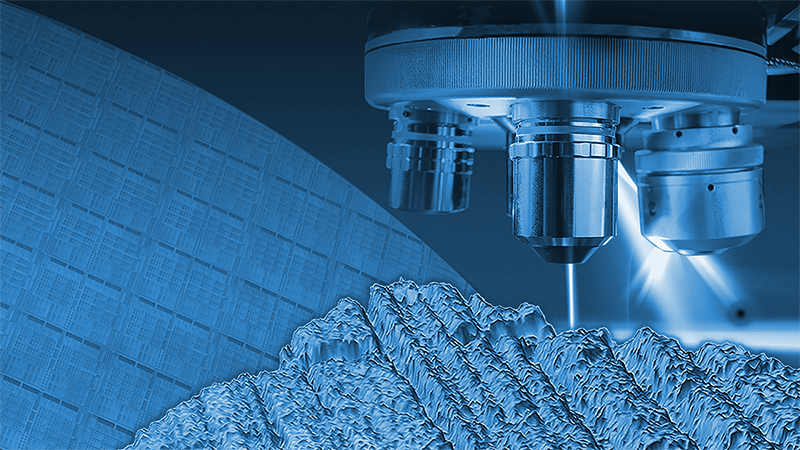
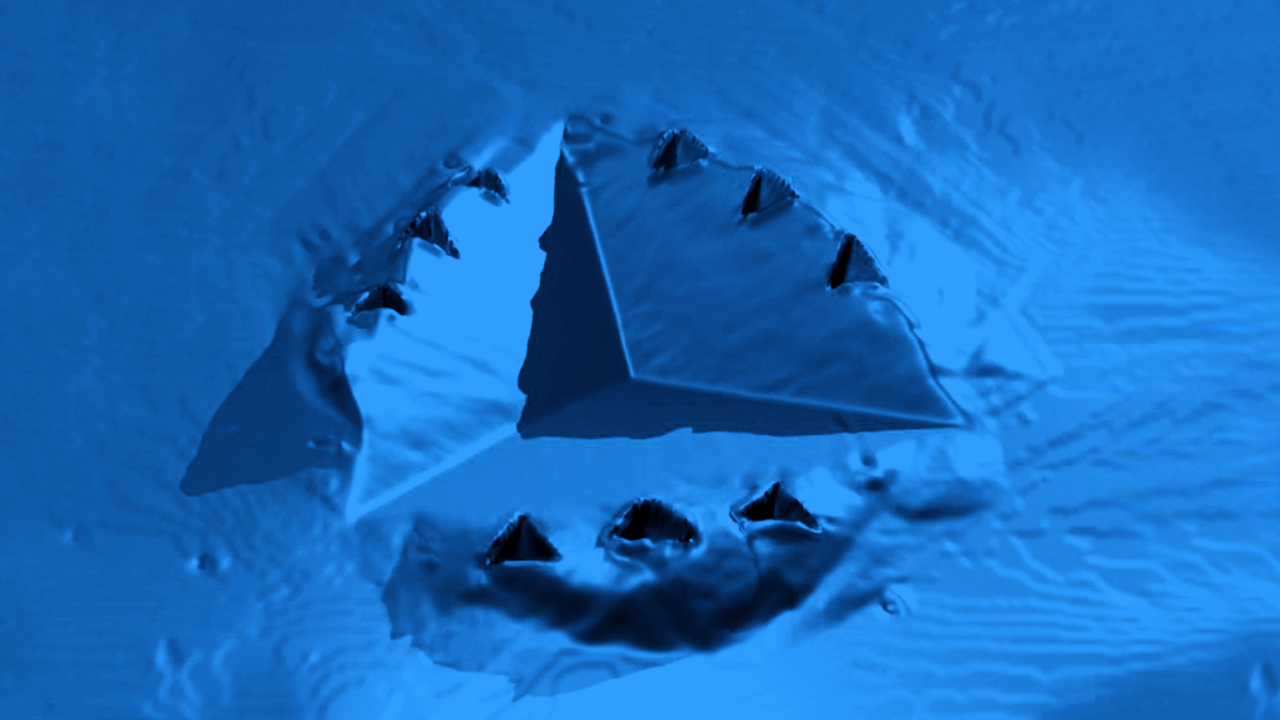
Quantify microscale finish, radius of curvature variations, scratches, and other defects
Orthopedic implants vary enormously. These variations can be in size, from tens of centimeters to millimeters; in shape, from simple spherical femoral heads to complex saddleshaped knee prostheses; in material, from stainless steel to hydroxyapatite; and in surface finish, from super smooth for reduced friction to intricately textured to promote stability. With such a wide range of parameters, control of the critical specifications of a part can become a challenge involving many different metrology instruments suited to different tasks.Bruker’s 3D optical profilers measure surface roughness in seconds with subnanometer repeatability and accuracy over large fields of view to quantify microscale finish, radius of curvature variations, scratches, and other defects that affect the tribological behavior of implants. The result is maximized lifetime for a wide variety of implants, from hip balls and cups to knees, elbows, and spinal implants.
Mechanical Properties
Measure mechanical, tribological, and interfacial adhesion properties critical to product reliability
Materials development has had a fundamental impact on the enhanced performance and capabilities of medical implants and devices. Materials utilized for most medical applications require a complex combination of unique mechanical and tribological properties, physical attributes, and biocompatibility. Mechanical and tribological properties are a critical design consideration for product performance and reliability, while the in-vivo preservation of properties can serve as an indicator of biocompatibility.
Bruker has developed a comprehensive suite of testing techniques to measure the mechanical, interfacial, and tribological performance of materials used in medical implants and devices over the nanometer and micrometer length scales. These nanoindentation, microindentation, and tribological characterization techniques allow scientists and engineers to quantitatively measure and optimize localized properties, resulting in enhanced product performance and reliability.