MALDI Biotyper® sirius System
The MALDI Biotyper® sirius System: unveiling new horizons in microbial analysis
As a global leader in MALDI-TOF mass spectrometry, Bruker continually enhances the functionalities of its comprehensive solutions. The MALDI Biotyper stands as a cornerstone technology, adeptly tackling the escalating challenges encountered in modern microbiology laboratories. This reliable tool is tailored to address the distinctive requirements of various microbiological market segments, spanning clinical, pharmaceutical, and diverse industries. Simultaneously, it paves the way for pioneering scientific exploration.
without any extra cost or effort
Enhanced capabilities
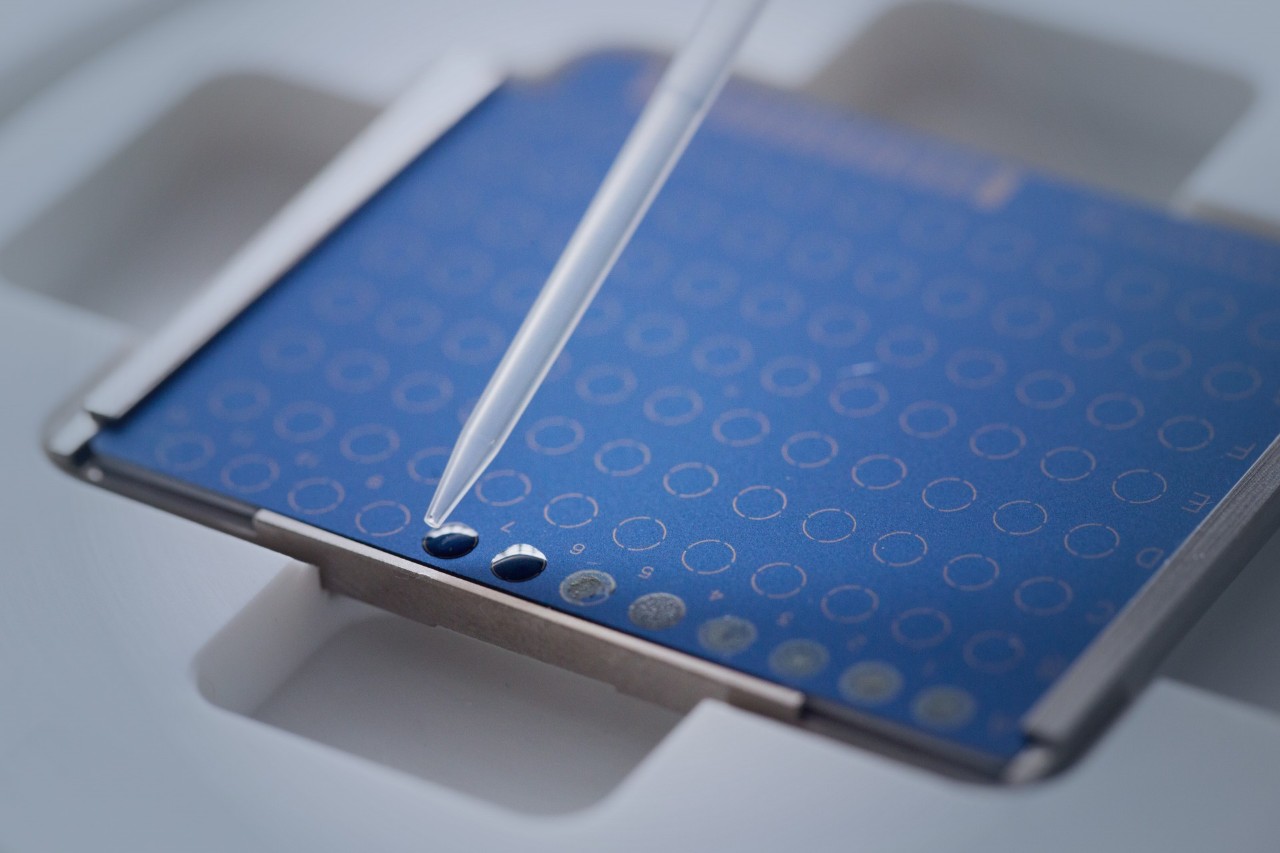
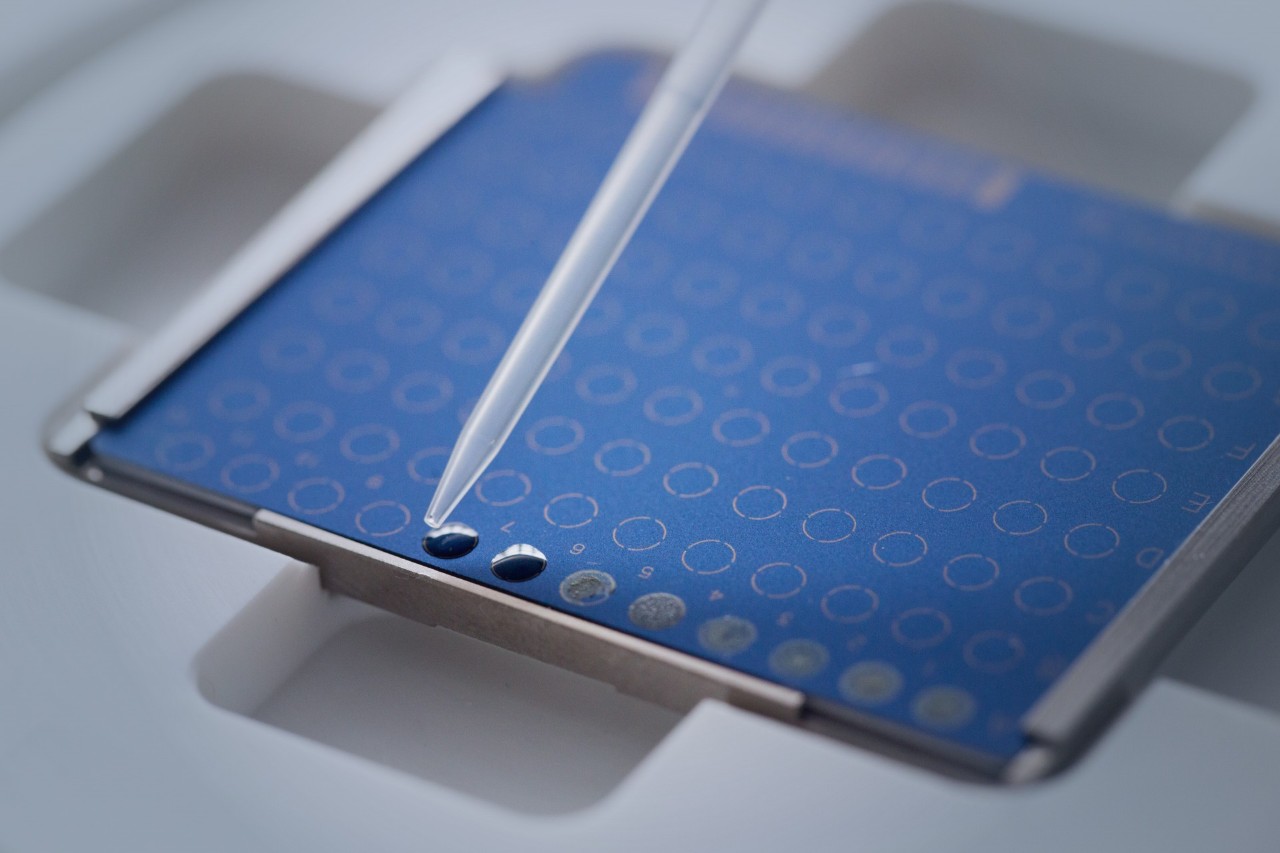
The Bruker MALDI Biotyper sirius System harnesses the cutting-edge technology that has made its predecessors so highly regarded. Equipped with a high-capacity vacuum system and a rapid smartbeam™ lifetime* laser, this system is primed for exceptional operation.
*Lifetime refers to 7 years or 500 million shots, whichever comes first.
** Negative ion mode is for Research Use Only
The system's capabilities have been broadened, enabling seamless positive and negative** ion detection. This expansion opens doors to a wider range of applications and research possibilities.
Lighter, faster, and more intuitive
Weighing in at a mere 75 kg (165 lbs), the MALDI Biotyper sirius System is a true benchtop marvel. Its compact design facilitates effortless integration into any laboratory setup, including LIS-connection. The system's user-friendly workflows and features including rapid target exchange and an integrated LED strip, provide enhanced convenience and efficiency in your daily operations.
The intuitive MBT Compass HT Software packages have been designed to enhance the performance and security of your MALDI Biotyper System, while bringing the system’s speed and efficiency to new heights, enabling fast analysis of up to 600 samples per hour. MBT Compass HT reassures the MALDI Biotyper sirius System’s performance being kept at top level with the novel zero-button IDealTune™ feature - the perfect solution for effortless performance optimization, without any extra cost or effort.
Positive/Negative Ion Mode: unveiling new dimensions and supporting collaborative progress
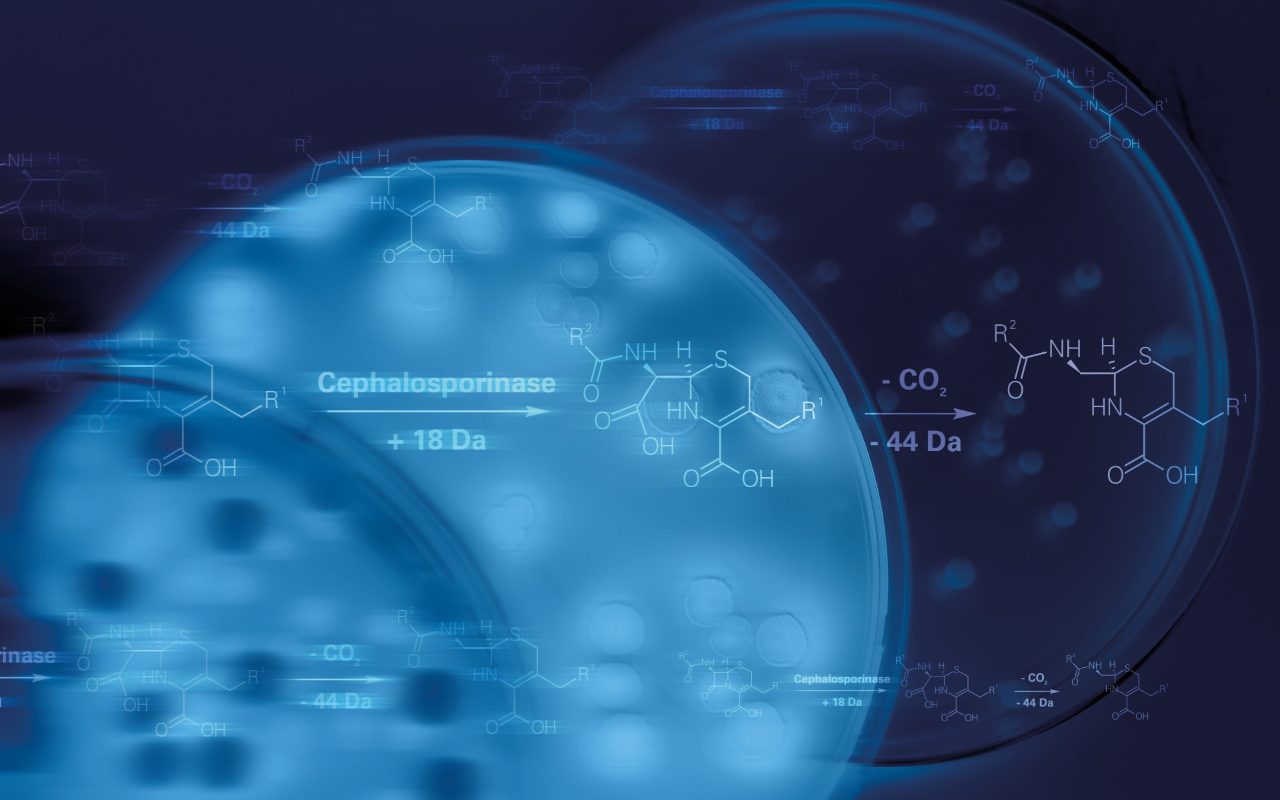
Our revolutionary MALDI Biotyper sirius System is not limited to routine microbial identification. While positive ion mode remains a cornerstone for everyday analysis, the introduction of negative ion mode opens up exciting avenues in microbial research. Dive into cutting-edge lipidomics investigations, uncovering new insights and rapid detection techniques for e.g. resistance-related modifications of lipid A in colistin-resistant bacteria. 1,2
Join the ranks of visionary researchers who are revolutionizing microbial analysis. With the MALDI Biotyper sirius System, you gain access to a world of possibilities.
¹Dortet L et al., 2018, Scientific Reports, Vol: 8, ISSN: 2045-2322
²Dortet L et al., 2018, Journal of Antimicrobial Chemotherapy, Vol: 73, Pages: 3359-3367, ISSN: 0305-7453
A solution for every microbiology lab
You want to equip your lab with eco-friendly instruments? Our MALDI Biotyper sirius System earned the ACT Label, a benchmark for sustainability, from manufacturing and daily operation to end-of-life disposal, combining top microbial identification performance with environmental responsibility throughout the product lifecycle.
The MALDI Biotyper sirius System is available in four different solution packages. Please contact your local representative for availability in your country:
MALDI Biotyper sirius IVD System
An IVD CE system for in vitro diagnostic identification of microorganisms originating from human specimens.
For professional use only. Not for sale in the USA.
MALDI Biotyper sirius CA System
Cleared USA FDA approval under Section 510(k) for the identification of gram-negative and gram-positive bacteria, anaerobic bacteria and yeast, cultured from human specimens.
Only for sale in the USA and Puerto Rico. For prescription use only.
-
MALDI Biotyper CA System (Brochure)
(PDF, 9 MB)
-
Expert Insights - Advancing Infectious Disease Testing with MALDI-TOF Mass Spectrometry
(PDF, 302 KB)
-
Expert Insights - Advanced Microbial Identification with MALDI Mass Spectrometry for Streamlined Therapeutic Treatment
(PDF, 551 KB)
- Library Organism List, Claim 6
MALDI Biotyper sirius RUO System
For microorganism identification in research applications.
For Research use only. Not for use in clinical diagnostic procedures.
MALDI Biotyper sirius GP System
For industrial applications such as Food, Veterinary, Water or Pharma environments.
Not for use in clinical diagnostic procedures.
-
MALDI Biotyper Pharma GP (Brochure)
(PDF, 3 MB)
-
MALDI Biotyper for Food Microbiology (Brochure)
(PDF, 6 MB)
-
MBT Compass HT, GP/RUO (Flyer)
(PDF, 280 KB)
-
Faster identification method for pharmaceutical microbiology with the MALDI Biotyper (Application Note)
(PDF, 339 KB)
-
Expert insight - The MALDI Biotyper® enables increased throughput and reliability in pharmaceutical microbial testing
(PDF, 1 MB)