生物制剂和生物仿制药的表征
相似性评估、高级结构、转化后调整、聚集、聚山梨酯和填充检查
生物制剂和生物仿制药,也被称为生物治疗药物,是由细菌、酵母和哺乳动物细胞等生物体产生的:这与小分子药物不同,小分子药物是通过化学方法合成的。这意味着,除了是非常大的分子(肽、小蛋白、抗体、多糖等)之外,它们还表现出转化后修饰和一定程度的结构变化。
通过结构表征确认治疗药物是什么,从主要氨基酸序列到高级结构,再加上杂质控制,是确保疗效和患者安全的关键因素。
布鲁克的质谱(MS)解决方案超越了传统的肽图和完整质量分析。这些解决方案将终极性能、速度和多功能性与强大的工作流程软件结合起来,为生物制剂开发商提供了可以随时使用的正交表征分析方法。从单克隆抗体(mAb)亚单位分析,使用中间向上和中间向下的方法,明确检测和表征序列变体和调整,包括脱酰胺、氧化和N端剪裁,到高级结构研究,详细表征二硫桥、构象表位、扰动后的结构变化,到计算抗体药物结合物(ADC)研究中准确的药物抗体比率(DAR)。
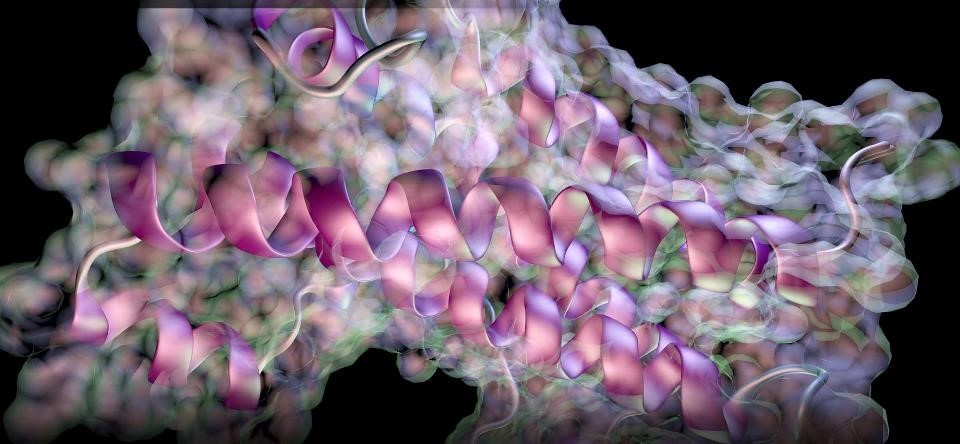
二硫键(DSB)分析和氢氘交换(HDX)已经成为可用于深入了解蛋白质结构的技术。这些见解对于确定生物仿制药的结构相似性或在药物研发期间监测蛋白质的稳定性至关重要。治疗性蛋白质的三级结构是其活性和稳定性的关键。
科研实验室需要能够自动分析生物制药中的DSB的技术,基于对未还原蛋白质的单一消化,并且不需要事先了解酶的特异性或本地DSB。由于这些蛋白质的复杂性和它们将包含多个二硫键的事实,分析是一个挑战,往往需要对还原和非还原蛋白质的胰蛋白酶消化物进行几次LC-MS运行,并对这两次分析进行手动比较。
Solutions
NMR is especially sensitive to changes to higher order structure at atomic resolution, making it ideally suited for similarity assessment of biologics and biosimilars. NMR allows for intact protein analysis, enabling structural evaluation of therapeutic drugs without modification, in conditions that are physiologically relevant. The selectivity and quantitative nature of magnetic resonance means that potency determination, impurity profiling and degradation studies (eg polysorbates) can be performed directly enabling fast and easy testing without the need for response factor calculations or the method redevelopment activities required by traditional LC methods, thereby saving time and reducing costs. In addition, benchtop time domain NMR (TD-NMR) is becoming an important technique for the analysis of protein aggregates and 100% fill-check of vials and syringes.
Fourier Transform Infrared (FT-IR) spectroscopy can be used to analyze water-soluble and membrane proteins such as nuclear receptors, which are currently decisive targets in drug research and development, being associated with conditions such as Alzheimer's, and Parkinson's disease, diabetes and obesity. FT-IR is typically applied in protein secondary structure elucidation, detection of conformational changes and monitoring of protein dynamics like aggregation, precipitation and crystallization. Infrared protein analysis is a quick and relatively inexpensive technique used for formulation optimization, stability studies during drug development and QC of protein drug products.
Surface Plasmon Resonance (SPR) is the gold standard methodology to characterize an interaction from a kinetic point of view. While solution-based approaches offer an equilibrium-based perspective on an interaction, the label-free and real-time analysis of SPR affords insight into the kinetics a key characteristic that enables more precise understanding of an interaction. Biologics demonstrating similar affinities using equilibrium-based methods like ELISA may have vastly different off-rates with subsequently different biological outcomes. SPR however enables the identification of biologics with the optimal half-life at your target protein.
SPR also allows researchers to identify antibodies that target different epitopes on a protein. Equally, conditional binding based on factors like pH, e.g. as in cancer tissue, or the effect of different buffers on the binding interaction can be investigated with ease. These approaches help to understand an interaction in a physiologically more translatable context.
解决方案
核磁共振(NMR)对原子分辨率的高级结构变化特别敏感,因此非常适合于生物制剂和生物仿制药(高级结构)的相似性评估。NMR可以进行完整的蛋白质分析,使其能够在与生理相关的条件下对治疗药物的结构进行评估,而无需任何调整。由于磁共振的定量特性及其选择性,直接进行药效测定、杂质分析和降解研究(如聚山梨酯),实现快速和简单的测试,无需反应因子计算,也不需要传统LC方法所要求的方法重建活动,从而节省时间和降低成本。此外,台式时域核磁共振(TD-NMR)正在成为分析蛋白质聚集和小瓶和注射器100%填充检查的一项重要技术。
傅立叶变换红外光谱(FT-IR)可用于分析水溶性和膜蛋白,如核受体,它是目前药物研究和开发的一个非常重要的目标,与阿尔茨海默病、帕金森病、糖尿病和肥胖症等疾病有关。FT-IR通常应用于蛋白质二级结构的阐明、构象变化的检测和蛋白质动态的监测,如聚集、沉淀和结晶。红外线蛋白质分析是一种快速和相对便宜的技术,用于配方优化、药物开发期间的稳定性研究和蛋白质药物产品的质量控制。
Surface Plasmon Resonance (SPR) is the gold standard methodology to characterize an interaction from a kinetic point of view. Whilst solution-based approaches offer an equilibrium-based perspective on an interaction, the label-free and real-time analysis of SPR gives insights into the kinetics and thus adds a key characteristic to understand an interaction more precisely. Biologics with similar affinities in equilibrium-based methods like ELISA may have vastly different off-rates with subsequently different biological outcomes. SPR thus enables to identify the biologics with the optimal half-life at your target protein.
SPR further allows to identify antibodies targeting different epitopes on a protein. Equally, conditional binding based on factors like pH, e.g. as in cancer tissue, or the effect of different buffers on the binding interaction can be investigate with ease. These approaches help to understand an interaction in a physiologically more translatable context.