Oncology
Advancing Discoveries for Cancer Treatment with Preclinical Research
A “one size fits all” approach is not suitable for cancer treatment, as
there are many different tumor types, some of which we don’t know much
about. Imaging technologies help us study the mechanisms of tumor
progression and the effects of treatment
There have been
tremendous advances in cancer treatment over recent years; however there
are still a number of tumor types that are not well understood and do
not respond optimally to available treatments. Furthermore, there are
many more cases for which treatment is not curative or causes severe
side effects. Consequently, research continues to discover new cancer
treatments and develop ways to better target current treatments. Tumor
imaging techniques continue to play a pivotal role in such research
endeavors.
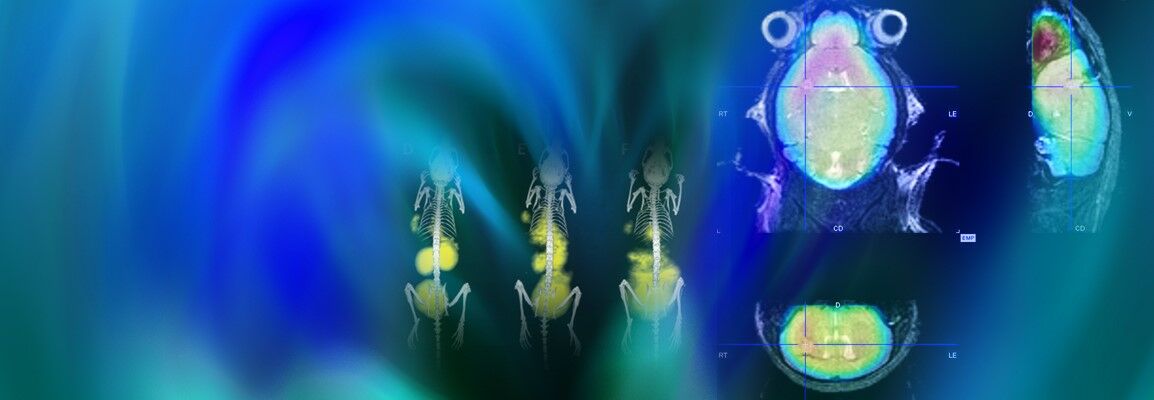
Non-invasive imaging provides key insights into tumor development, response to treatment and drug toxicity. The analytical value of individual imaging strategies can be further enhanced by combining structural and functional imaging modalities. Multimodal imaging in preclinical research using in vivo imaging allows cancer-related processes to be visualized in real time for extended durations, with high sensitivity and resolution. A deeper understanding of a range of tumors and improved treatment options have been obtained through the use of a variety of imaging techniques, including MRI, SPECT, PET and CT, and microCT.
Bruker provides a full range of innovative non-invasive in vivo imaging technologies to facilitate the understanding of the course and mechanisms of tumor progression and the effects of treatment. Dual-modality imaging configurations like PET/CT and PET/MR or even tri-modal instruments that combine PET, SPECT an CT technologies, address today's most challenging research needs,and provide quantitative 3D tomographic images of radiotracers, bone, and soft tissue.