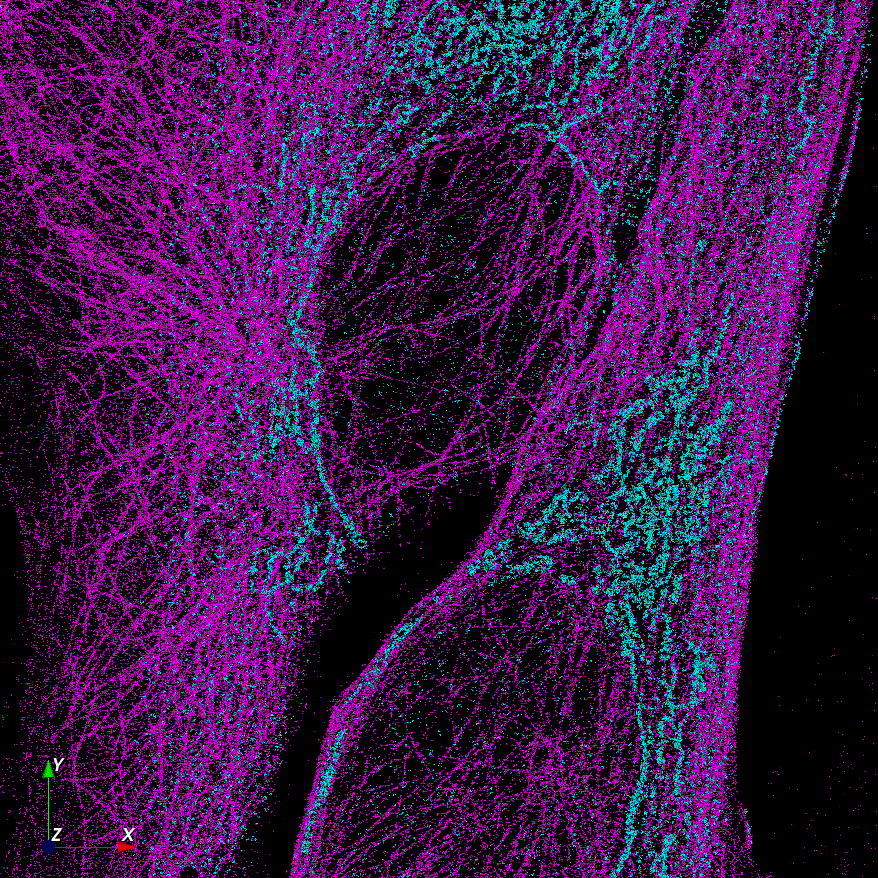

Advanced Two-Color Super-Resolution Microscopy with Photon Reassignment
Simultaneous two-color imaging with the Bruker Vutara VXL super-resolution microscope offers significant advantages for advanced biological research. This white paper describes the key features of the Vutara VXL's dual-camera solution, which benefits scientists in cell biology, neuroscience, chromatin tracing, and other disciplines.
Key advantages include:
- Faster Data Acquisition: Simultaneous imaging halves acquisition times, essential for high-throughput studies and time-sensitive experiments.
- Improved Co-localization Accuracy: Minimizes motion artifacts, enhancing live cell imaging and single particle tracking (SPT) accuracy.
- Enhanced Study of Biomolecular Interactions: This technology enables real-time observation of dynamic interactions between different biomolecules, providing deeper insights into cellular processes.
Overall, the Vutara VXL’s dual-camera setup increases the efficiency of data collection and enhances the reliability and resolution of super-resolution microscopy studies.
Precision Imaging for Advanced Cell Biology
Simultaneous two-color imaging with the Bruker single-molecule localization super-resolution microscope (SMLM) Vutara VXL offers significant advantages for advanced biological research. Firstly, it enables faster data acquisition, which is crucial for high-throughput studies and time-sensitive experiments. Secondly, minimizing motion artifacts improves co-localization accuracy, particularly in live cell imaging and single particle tracking (SPT). Additionally, simultaneous imaging of two colors enhances the ability to study dynamic interactions between different biomolecules in real time, providing deeper insights into cellular processes. Overall, this approach increases the efficiency of data collection and enhances the reliability and resolution of super-resolution microscopy studies.
Dual-Camera System for Simultaneous 3D Two-Color Imaging
Equipped with two cameras, the system splits the emission into two wavelength bands, each detected by a separate Orca Fusion BT sCMOS camera. These configurations enable simultaneous detection of two colors, effectively halving acquisition times. Software algorithms align both cameras, facilitating super-resolution co-localization experiments. Operating at frame rates exceeding 100 Hz, these cameras support two-color single particle tracking (SPT) of fast-moving targets in living cells.
The Bruker Vutara VXL utilizes a unique bi-plane design to detect the 3D position of each emitter within a z-range of up to 1000 nm. A high-resolution piezo focus drive allows the stacking of these 3D slices to create true 3D z-stacks, akin to confocal microscopy but with the enhanced resolution of single-molecule localization microscopy (SMLM). The dual-camera setup extends this capability to acquire two-color z-stacks simultaneously.
Motorized Filter Wheel for Rapid Sequential Imaging of Additional Dye Pairs
In cases where two colors are insufficient, a motorized fast filter wheel enables switching bandpass filters to image additional dye pairs. This filter wheel is seamlessly integrated into the workflow-driven Vutara SRX software. Combined with Bruker's PlexFlo microfluidic solutions, this setup supports sophisticated multiplexing experiments, including multi-target immunofluorescence, omics applications, exchange-PAINT, and chromatin tracing.
Emission Ratio Measurement Prevents Bleed-Through
Red dyes are advantageous for SMLM experiments due to their superior blinking characteristics and the reduced phototoxicity of long-wavelength excitation light. In two-color experiments, red dyes offer more compatibility with the same blinking buffer for dSTORM experiments than other wavelengths. Dyes with similar spectra are particularly beneficial for co-localization experiments, as they exhibit minimal chromatic shift.
In traditional microscopy, dyes with overlapping emission spectra are avoided due to crosstalk. However, SMLM leverages ratiometric imaging to distinguish signals from two dyes by recording them simultaneously in separate spectral channels. Since SMLM localizes signals from individual dye molecules, the intensity ratio in two spectrally separated channels depends solely on the dye’s spectrum, not on its concentration, unlike in widefield, confocal, SIM, or STED microscopy.
The Bruker Vutara VXL's dual-camera solution effectively uses ratiometric detection to separate signals from dyes with overlapping emission spectra.
Photon Reassignment for Enhanced Localization Accuracy in Dual-Camera SMLM
In standard multi-fluorescence microscopy, photons with wavelengths outside the emission filter’s transmission band are typically lost, leading to reduced localization accuracy and resolution in SMLM experiments. The Bruker Vutara VXL addresses this limitation by fitting measured point spread functions (PSF) to dye emissions detected on both cameras. This dual-camera fitting approach recovers otherwise lost photons, enhancing localization accuracy and overall resolution.
User-Configurable Dichroic and Filters for Enhanced Flexibility
The Vutara VXL supports up to five single and three dual band-pass filters, accommodating most experimental needs. In cases where additional configurations are required, such as developing new dyes, users can easily exchange the dichroic that splits the emission between the two cameras. Additional filters can be inserted in front of the cameras, providing further customization options.


