
NMR Spectroscopy Aids GPCR Drug Discovery
Advances in molecular biology have furthered the understanding of the etiology of many disorders and knowledge of the three-dimensional structures of therapeutically relevant targets. Such information is now widely used to inform rational structure-based drug design.
Molecular biology studies have identified and characterized numerous potential binding sites for inhibiting or augmenting specific biochemical pathways for therapeutic effect. These formed the foundation for structure-based drug design.
Drug design refers to the development of a therapeutic agent that explicitly targets a selected binding site on, for example, an enzyme, or receptor protein, to achieve the desired effect to correct a dysfunctional pathway and reduce disease burden.
Drugs developed in this way are typically small organic molecules, such as protein fragments, that are complementary in shape and charge to the biomolecular target. Computer modeling techniques are often used to determine the required structure and analytical technologies, such as nuclear magnetic resonance (NMR), are used to screen for potential drug candidates.
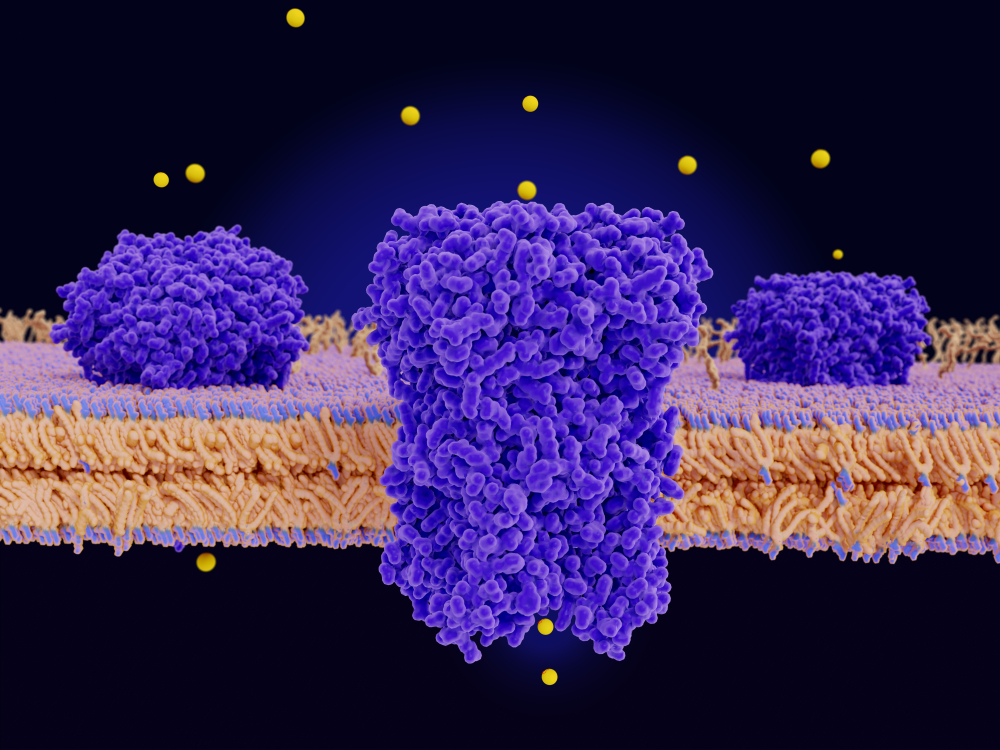
G protein-coupled receptors (GPCRs) have proved a popular target for structure-based drug design since they regulate several key physiological processes. They are one of the largest and most diverse mammalian protein families.
These transmembrane receptors transduce extracellular stimuli into intracellular signals that are fundamental for most physiological processes, including vision, and a range of neurological, cardiovascular, endocrine, and reproductive functions.
Three-dimensional structures have been determined for more than 50 different human GPCR proteins, providing a solid platform to support targeted drug development. Indeed, more than 30% of all FDA-approved drugs target a GPCR for a range of indications, including inflammation and diseases of the central nervous system as well as cardiovascular, respiratory, and gastrointestinal disorders.
However, being such a large family of receptors, GPCRs still have considerable potential as targets for novel therapeutic agents. To realize this potential, additional detailed knowledge of the complex structure-function relationships involved in GPCR signaling is needed.
Nuclear magnetic resonance (NMR) spectroscopy has been identified as a key approach for extending the GPCR knowledge base and facilitating the design of new GPCR-targeted drugs. Traditionally, x-ray crystallography has been used to elucidate GPCR-drug complex formation. However, NMR offers the benefits of evaluating GPCR complexes in solution at physiological temperature and requiring minimal modification of GPCR covalent structures. NMR thus enables the study of dynamic features relating to the interaction between GPCRs and potential drug candidates.
Numerous NMR studies of GPCRs have already complemented the structural knowledge acquired using other biophysical techniques and highlighted additional opportunities for drug discovery.
In the future, NMR may facilitate the realization of such opportunities through rapid, high throughput screening of protein fragments to identify potential drug candidates. It can also be used in dynamic structure studies to further understand the interactions between a GPCR and its ligands, enabling the pharmacological characteristics of GPCR-targeted drugs to be optimized.

