Molecular Tracers and Contrast Agents
Imaging techniques rely on molecular tracers and ontrast agents to produce clear images
Medical imaging techniques such as positron emission tomography (PET), single photon emission computed tomography (SPECT), magnetic resonance imaging (MRI), magnetic particle imaging (MPI) and micro computed tomography (microCT) rely on the use of molecular tracers and contrast agents to generate, clear interpretable images. These imaging agents are used to acquire information on cells, organs, cancerous tissue and healthy tissue, to assess bodily functions, diagnose disease and monitor the effects of therapies.
The two most widely used modalities in nuclear medical imaging are PET and SPECT. These techniques require the use of radioactive tracers (radiotracers) to provide information on specific aspects of molecular activity such as tissue metabolism, for example. Dozens of FDA-approved radiotracers are currently available, and with hundreds being evaluated, tracer development is at the forefront of preclinical imaging research and many new agents are in the pipeline. Additionally, many conditions would benefit from more disease specific tracers and for certain diseases such as multiple sclerosis, no clinically approved tracers are available. Therefore, developing new tracers for the study and diagnosis of disease remains an important area of research.
Small animal studies are necessary to explore and validate imaging agents before beginning clinical trials. Preclinical SPECT and PET imaging is increasingly being used by researchers in this development phase as it provides data that can be extrapolated from animal to human studies.
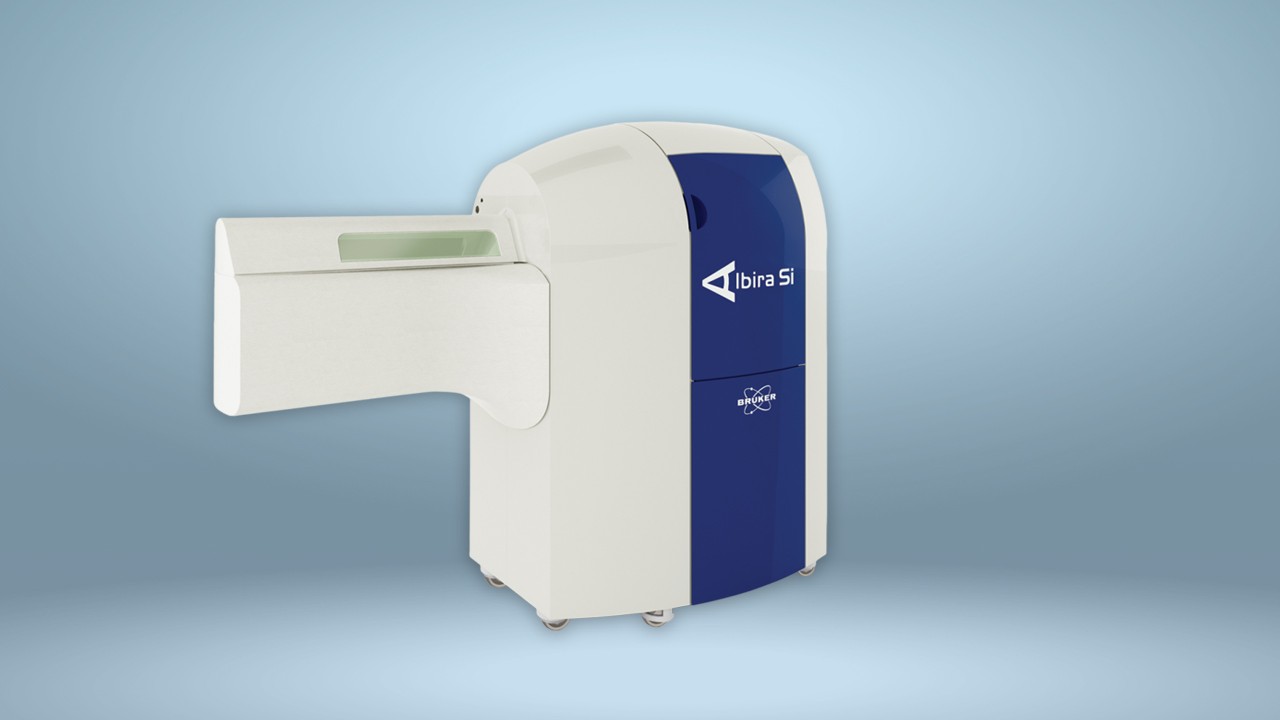
The AlbiraSi from Bruker is a combined PET, SPECT and CT system that provides quantitative 3D tomographic imaging of radiotracers, bone, and soft tissue. The system offers high resolution and extremely sensitive detection. The SPECT system can be used to image a wide variety of radiotracers and the CT system provides an accurate anatomical context for functional images of bone and soft tissue. The AlbiraSi is used by researchers to obtain in vivo data in preclinical experiments to accurately predict whether a radiotracer would be useful in the clinical setting. Bruker's instrumentation enables scientists to develop and evaluate the effectiveness of tracers in a range of live disease models, over time and with imaging methods that are directly translatable to clinical imaging.
Anatomical medical imaging modalities such as MRI and microCT require the use of contrast agents to improve visualization of tissues that would otherwise be difficult to distinguish such as the soft tissues of the nervous system, digestive system, breast, lung or liver.
Bruker's minispec Contrast Agent Analyzer enables researchers to characterize and validate the relaxation properties of contrast agents for use in MRI. The system is specifically designed to analyze the effects of these MRI contrast agents on the nuclear magnetic resonance (NMR) relaxation of fat or water protons. A contrast agent will always accelerate relaxation, thereby leading to negative or positive enhancement of contrast. MRI can be used to measure the relaxation times, but is relatively expensive to perform. Bruker has therefore provided the ideal solution in designing the minispec analyser as it provides the same magnetic field strengths as clinical MRI systems.
Support
Service and Life Cycle Support
Bruker’s commitment to provide customers with unparalleled help throughout the buying cycle, from initial inquiry to evaluation, installation, and the lifetime of the instrument is now characterized by the LabScape service concept.
LabScape Maintenance Agreements, On-Site On-Demand and Enhance Your Lab are designed to offer a new approach to maintenance and service for the modern laboratory