Sepsis and Resistance Testing
Sepsis and Resistance Testing
The threat of multi-drug resistance (MDR) among gram-negative and gram-positive pathogens has increased worldwide over recent years, impacting both hospital and community acquired infections. Bruker is addressing the need for rapid and accurate solutions to achieve fast identification and resistance detection from Positive Blood Cultures (PBC).
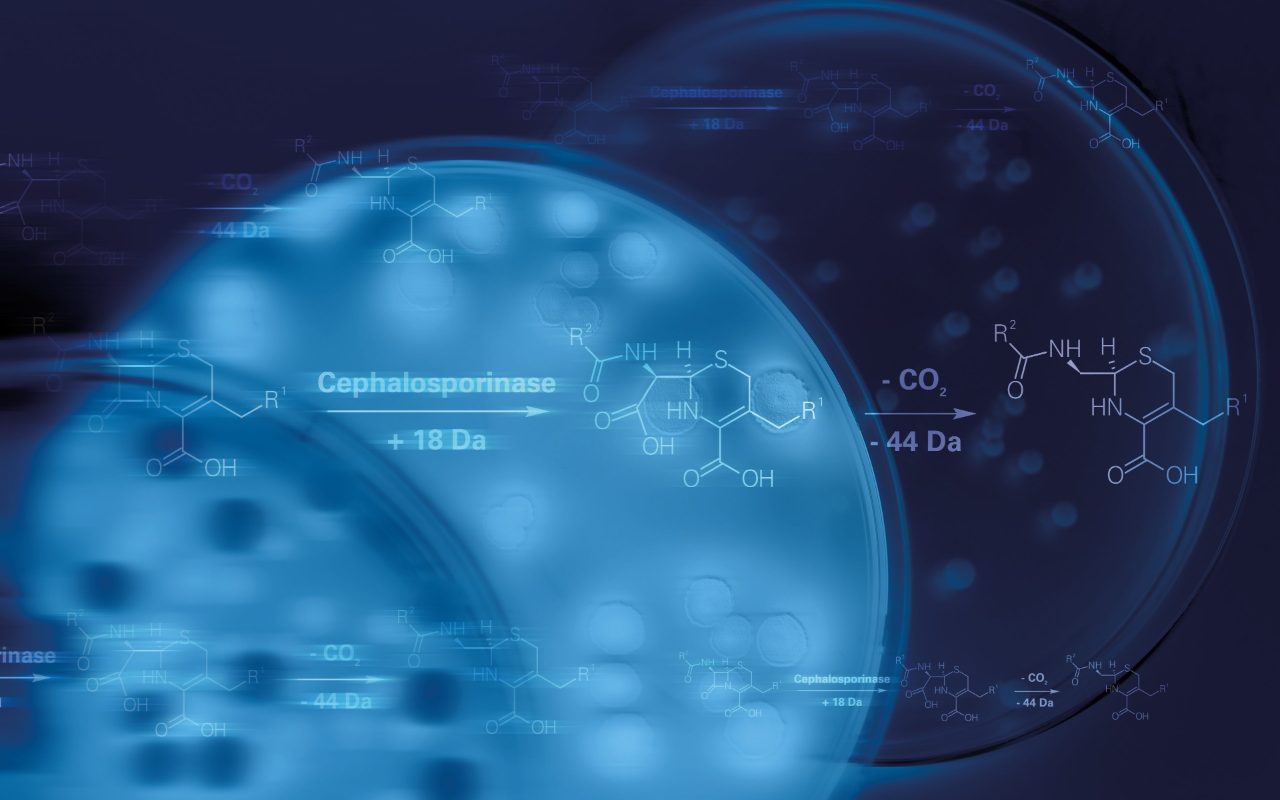
During the last decades, the MALDI Biotyper® has revolutionized microbial identification, offering a fast, easy, robust, reliable and cost-effective solution. As part of this product evolution, Bruker has focused efforts on finding novel solutions using the MALDI Biotyper® to shorten microbial identification. Starting from a PBC, actionable results can now quickly be obtained with the Sepsityper® solution (available for IVD-CE as well as US-IVD clinical workflows), followed by phenotypic resistance detection of carbapenemase and cephalosporinase, within 60-90 minutes after PBC alert, using the MBT STAR®-BL assays (only available for IVD-CE workflows).
After subculturing of the PBC, confirmation of resistance mechanisms and quantification of microbial resistance by determination of true MICs is performed using the MICRONAUT broth microdilution (BMD) product portfolio. MICRONAUT provides accurate, complete and true MIC of microorganisms as well as detection of important resistance mechanisms of Ambler class A (ESBL, KPC), class B (MBL), class C (AMP-C) and class D (OXA-48-like) cephalosporinases and/or carbapenemases.