ADME-Toxicology
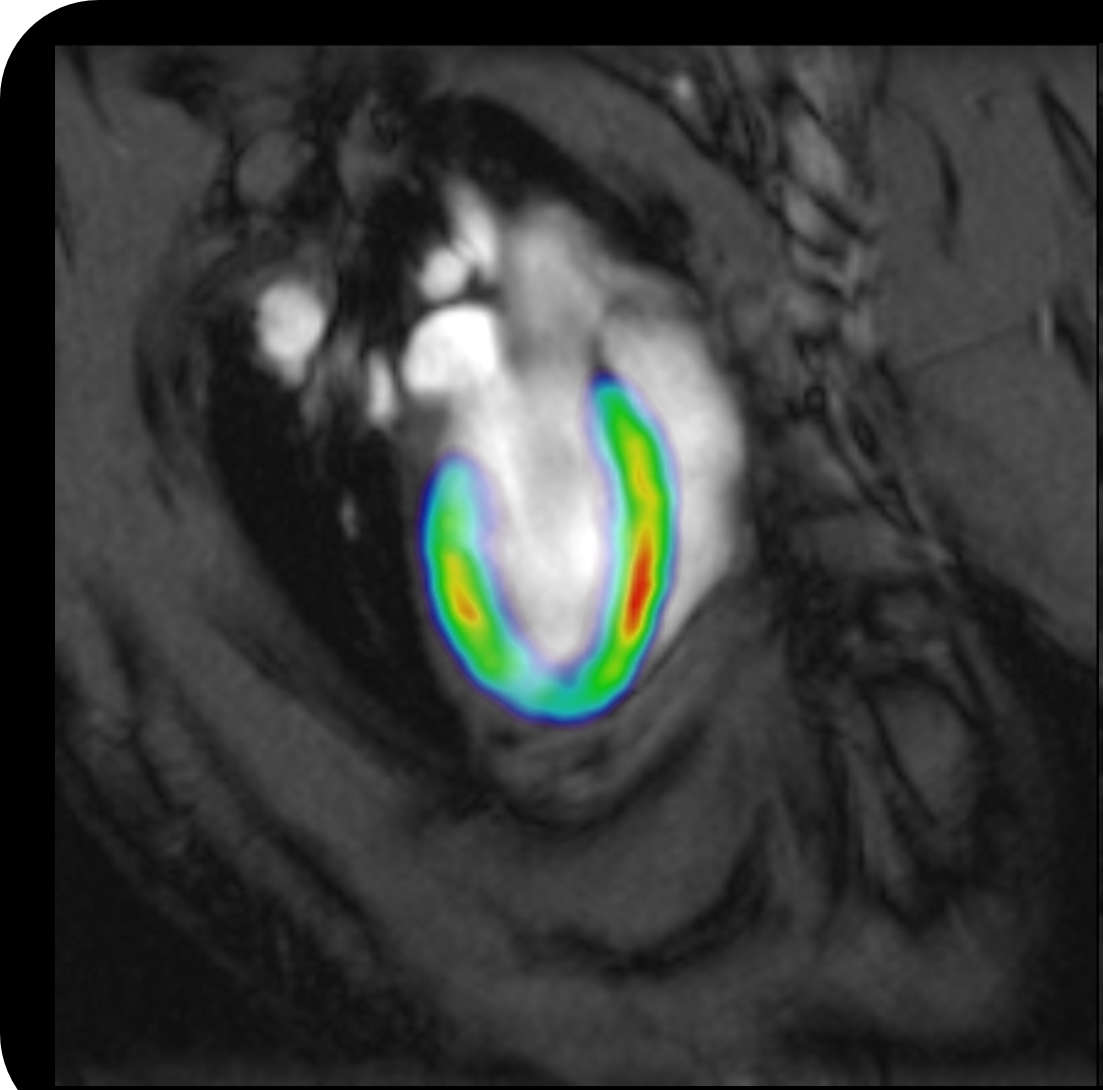
Imaging techniques such as MRI, PET, SPECT and micro CT can provide insights into safety liabilities through administration of the test compound in a longitudinal fashion. This allows the preclinical animal to serve as its own control and offer a method of non invasively understanding drug distribution profiles using techniques such as PET and SPECT to determine site of action with respect to on or off target effects. In combination with anatomical and functional parameters achieved using MRI, micro CT and PET it is possible to accurately determine efficacy vs the side effects associated with off target effects. Essentially, within the same animal, it is possible to determine biodistribution, efficacy and toxicity at the earliest opportunity either at the lead optimisation stage or during safety assessment conducted in life as opposed to the reliance on conventional time point analysis of ex vivo tissue.
In vivo imaging technologies are increasingly being employed to determine the major reasons for attrition, e.g. toxicity of liver, kidney, heart and brain as well as other organ tissue responses to novel therapeutics.
Completely standardized and fully automated nuclear magnetic resonance (NMR) metabolic profiling platform is the ideal and unique technology of choice for high-performance human bodyfluid analysis enabling effective clinical metabolomics research and related assay development (on Research Use Only Level)
LabScape
Service & Life Cycle Support for Magnetic Resonance and Preclinical Imaging
Bruker’s commitment to provide customers with unparalleled help throughout the buying cycle, from initial inquiry to evaluation, installation, and the lifetime of the instrument is now characterized by the LabScape service concept.
LabScape Maintenance Agreements, On-Site On-Demand and Enhance Your Lab are designed to offer a new approach to maintenance and service for the modern laboratory