From Single Molecules to Tissues: Application of Correlative BioAFM in Microscopy Core Facilities
Microscopy Core Facilities Benefiting from BioAFM
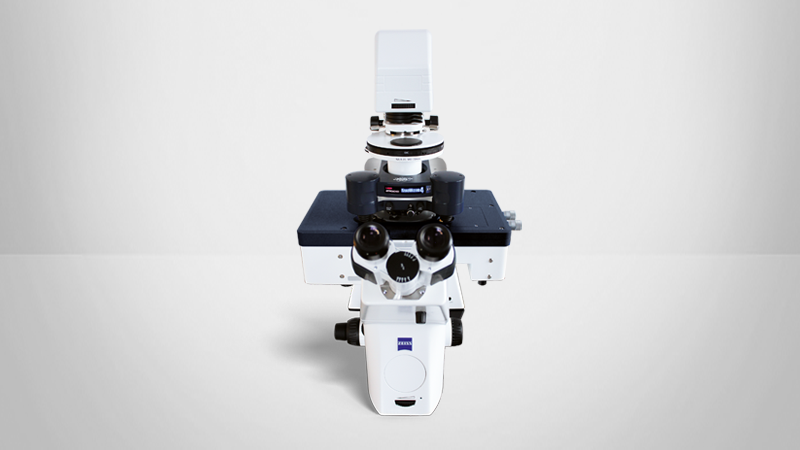
Observing the structure, dynamics, and function of biological samples and understanding the intrinsic relationship between them has become a key focus of modern biophysics and molecular biology. AFM is an advanced multiparametric imaging technique that delivers 3D profiles of sample surfaces and characterizes their nanomechanical properties. BioAFM allows the real-time, high-resolution investigation of complex biological samples and dynamic mechanisms under near-native conditions.
Bruker’s BioAFM platform can be seamlessly integrated with advanced optical microscopy techniques, such as confocal, fluorescence, and STED microscopy. The ability to obtain real-time, correlative data sets is particularly relevant in life science research. It is a powerful approach for investigating samples ranging from single-molecules and cells to tissues, organoids, and soft matter at the nanoscale, delivering valuable insights into the relationship between cell function, structure, and morphology at the nanometer scale.
This article outlines the capabilities of correlative AFM and its benefits for life science research in microscopy core facilities. Both AFM and light microscopy can be operated under ambient environmental conditions, enabling live-cell imaging and comprehensive sample analysis, leveraging the advantages of both techniques. Easy to use features make BioAFM ideal for both experienced AFM users and those new to the technique in multi-user imaging facilities.
Readers can expect:
- A brief overview of the AFM technique and BioAFM product platform
- Examples of BioAFM integrated with various optical microscopy techniques
- An outline of modes and features that facilitate the correlation of AFM and optical microscopy datasets
- Case studies outlining novel applications ranging from high-resolution molecular imaging to the visualization of highly dynamic processes in living cells and force mapping on osteoarthritic cartilage
KEYWORDS: Atomic Force Microscopy; Correlated AFM; BioAFM; AFM-STED; Mechanobiology; High-speed Correlative Microscopy;