UMIC® Product Line
Overcome the limitations of routine MIC testing methods
Ready-to-use unitary MIC testing solutions
Clinicians are lacking treatment options and must preserve the long-term efficacy of antibiotics. It is critical to obtain accurate AST results from the lab that they can trust.
Broth microdilution (BMD) is the reference method recommended by EUCAST and CLSI.
A flexible solution providing EUCAST & CLSI compliance, simplicity and convenient logistics is therefore required to answer these clinical needs.
Cost-effective drug testing
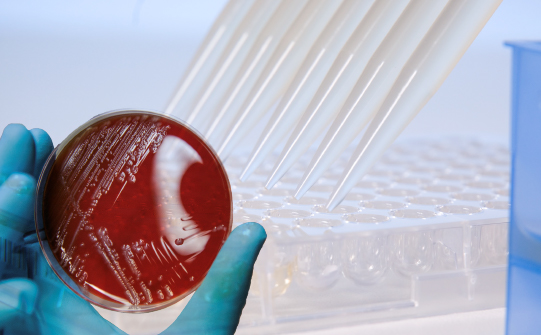
Test one or several drugs for a clinical isolate, from the same bacterial inoculum.
UMIC® Incubation boxes preserve a humid atmosphere and allow easy recognition in the incubator.
They also serve as a reading tool, with a reminder of the concentrations of the antibiotic present in the wells of the UMIC®.
Long shelf life and storage at room temperature simplify logistics and keep costs under control.
They are based on the broth microdilution (BMD) reference method recommended by EUCAST and CLSI.
Available for a range of antibiotics
UMIC® test kits are available for:
• Colistin
• Piperacillin-Tazobactam
• Vancomycin-Teicoplanin
• Daptomycin
• Cefiderocol
UMIC® Cefiderocol
Enabling antimicrobial susceptibility testing against the critically important and promising antibiotic cefiderocol in full compliance with EUCAST and CLSI recommendations by using iron-depleted Mueller Hinton broth. The UMIC® Cefiderocol is suitable to determine MIC values in the range of 0.03 to 32 μg/mL for Enterobacterales, Pseudomonas aeruginosa, Acinetobacter baumannii and Stenotrophomonas maltophilia.
UMIC® Colistin
Enabling antimicrobial susceptibility testing against the backup antibiotic colistin by using the broth microdilution method, as advised by EUCAST, for Enterobacterales and non-fermenting bacteria. BMD is currently the only valid method for MIC determination of colistin.
Colistin concentration range – 0.0625 to 64 mg/L
UMIC® Piperacillin-Tazobactam
Enabling antimicrobial susceptibility testing against the antibiotic-inhibitor combination piperacillin-tazobactam by using the broth microdilution method for Enterobacterales and non-fermenting bacteria.
Piperacillin-Tazobactam concentration range – 0.125/4 to 128/4 mg/L
UMIC® Vancomycin/Teicoplanin
Enabling antimicrobial susceptibility testing against the glycopeptide antibiotics vancomycin and teicoplanin by using the broth microdilution method, as advised by EUCAST, for Enterococcus faecalis and Enterococcus faecium positive for vanA/vanB, as well as Staphylococcus spp.
Vancomycin concentration range – 0.25 to 4 mg/L
Teicoplanin concentration range – 0.25 to 8 mg/L
UMIC® Daptomycin
Enabling antimicrobial susceptibility testing against the antibiotic daptomycin by using the broth microdilution method for Enterococcus spp. and Staphylococcus spp.
Daptomycin concentration range – 0.03 to 32 mg/L
UMIC® - Offering high accuracy single drug MIC testing
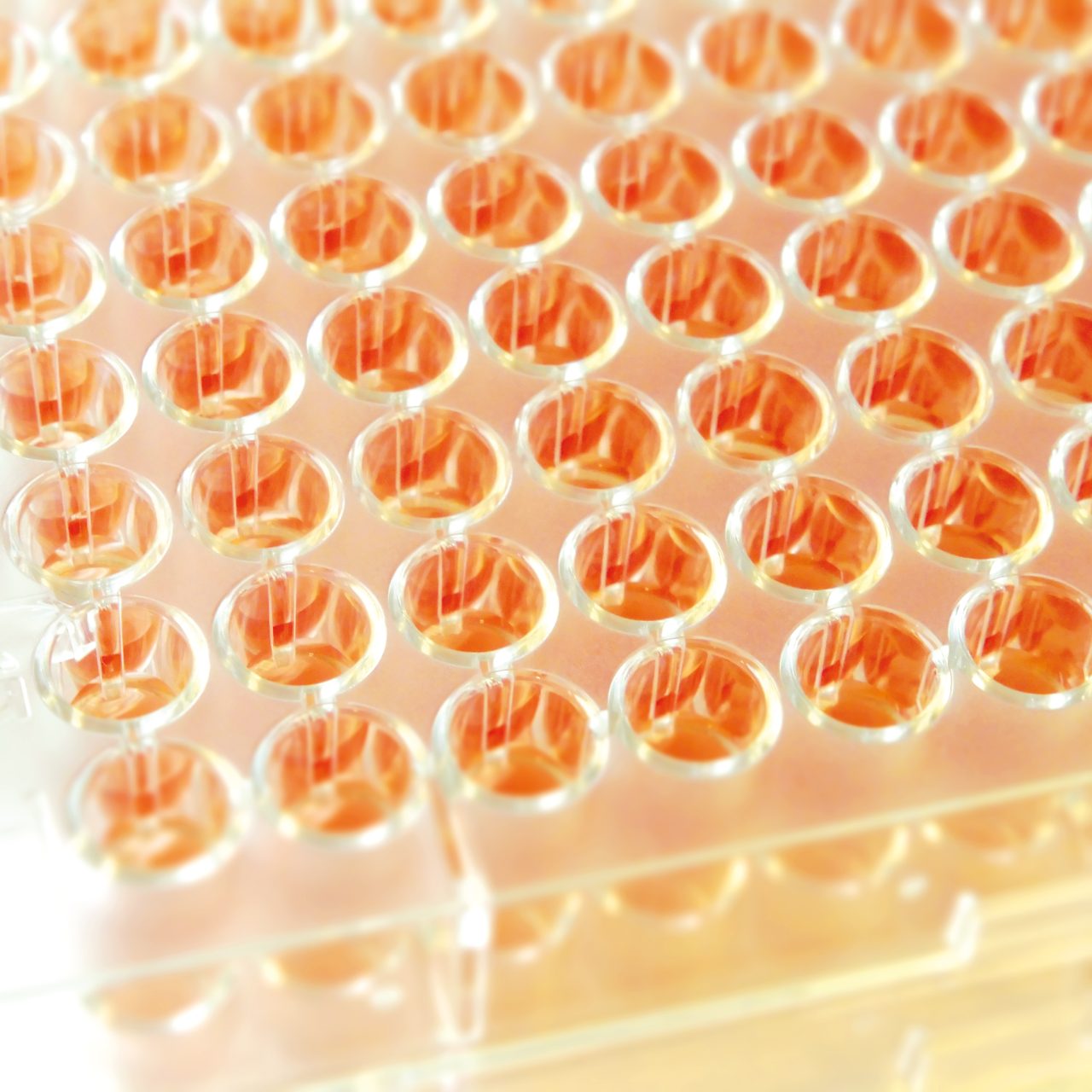
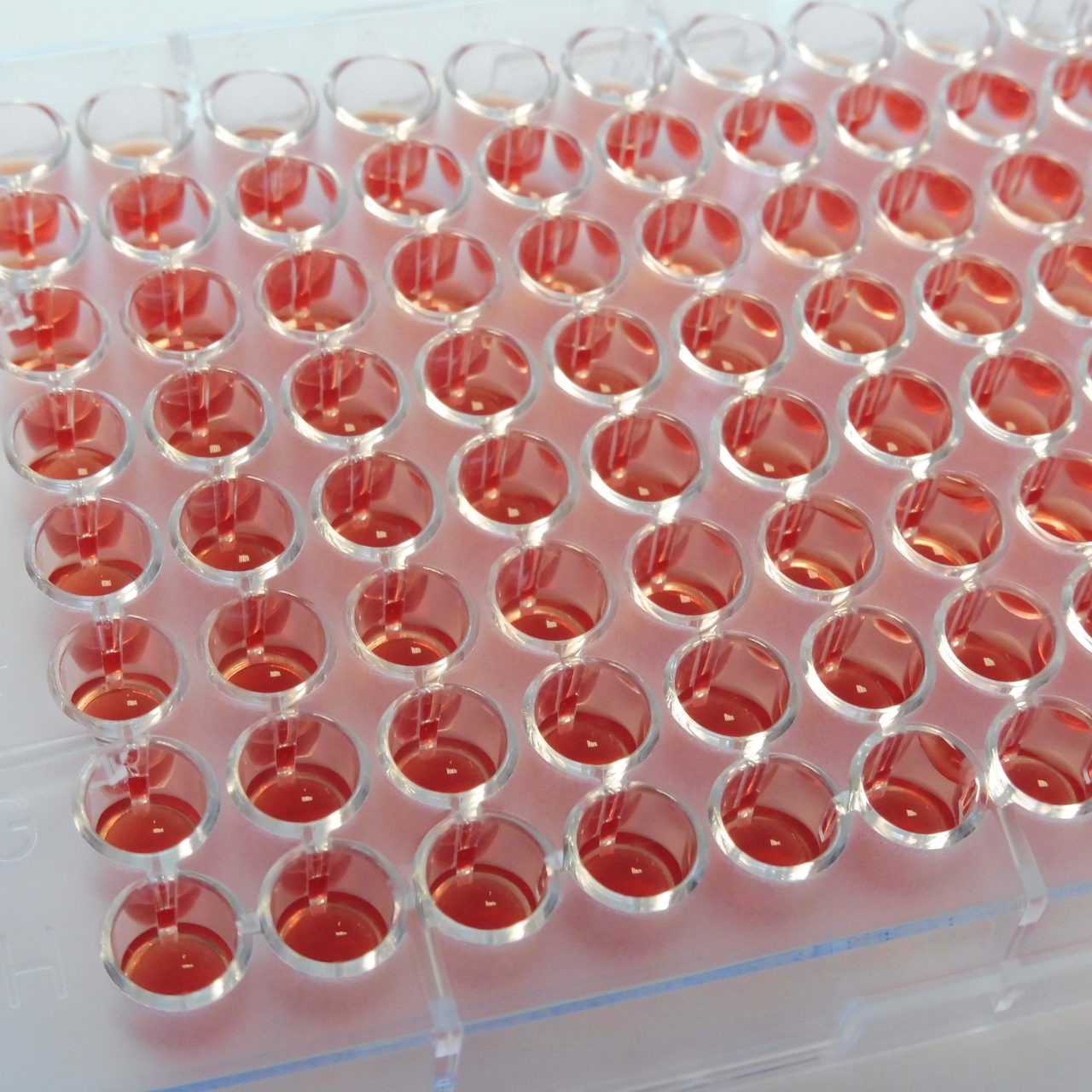
UMIC are ready-to-use strips with 12 wells each, containing dried antibiotics and prepared according to the ISO 20776-1 standard, therefore delivering accurate MIC results based on the reference method. As single strips, UMIC allow flexibility in the choice to test one or several drugs for a clinical isolate, from the same bacterial inoculum. The strips are clearly marked to avoid mixing up.
UMIC strips are easy to implement in routine by using the same 0.5 McFarland inoculum as prepared for general AST methods. The incubation boxes help maintain the humid atmosphere and can easily be recognized in the incubator. They also serve as a reading tool, with a reminder of the concentrations of the antibiotic present in the wells of the UMIC strip. The long shelf life (24 months from date of manufacturing) as well as convenient storage at room temperature are simplifying logistics and keeping costs under control.
Please contact your local representative for availability in your country.
Not for sale in the USA.