Application Note: Automated High-Content Screening for Drug Discovery with the Acquifer IM
Accelerated and Effective Drug Discovery
In this application note, readers will learn about Bruker’s Acquifer Imaging Machine (IM) — a fully automated widefield microscope with unique imaging capabilities and features that are ideal for high-content screening (HCS) and drug discovery assays. Discover its value for experiments, such as drug screenings, which are important for identifying therapeutic compounds and testing their efficacy and possible side effects.
Contents include:
- The benefits of automated imaging workflows for high-content screening
- How the Acquifer Plate-Viewer software is used for intuitive, high-throughput region selection
- Other scalable data processing and visualization solutions
- Application examples in zebrafish HCS, kidney disease modeling, and organoid-based drug discovery
Bruker’s Acquifer Imaging Machine (IM) is a fully automated widefield microscope with brightfield and fluorescence imaging capabilities. It is ideal for high-content screening (HCS) and drug discovery assays. The Acquifer IM’s static sample holder and mobile optical unit ensure sample stability during imaging. This is particularly crucial for motion-sensitive samples, including non-adherent cell cultures or embryos. The system also includes a host of features to enhance its utility and customization in automated workflows for drug discovery, including built-in temperature regulation, a robotic lid, and an open software interface. This application note discusses how these unique features and seamless setup of automated workflows facilitate advanced time-lapse experiments, screening, and high-throughput imaging assays.
Drug Screening Experiment Design
Drug screening is a crucial step in drug development. It helps to identify potential therapeutic compounds, evaluate their efficacy, and assess possible side effects before proceeding to clinical trials. Effective drug screening can save time and resources, ensuring that only the most promising candidates move forward.
An ideal setup for drug screening allows simple administration of drugs. Drugs can be added to water for zebrafish or screens using 96-well plates to enable high-throughput analysis. Both of these experimental setups are supported by the Acquifer IM, which also uses moving optics and tight environmental control to provide sample protection. The Acquifer IM was developed closely with active researchers to be an ideal setup for HCS drug screens, including popular models such as cell cultures, organoids, and zebrafish.
LEARN MORE:
Application Example: Zebrafish HCS
In zebrafish HCS setups, efficiently managing many samples is essential. Typical zebrafish screens include hundreds to thousands of samples, where larvae are studied in bulk in 96-well microtiter plates. However, samples must be consistently aligned to effectively analyze the phenotypic impact of drugs. Random orientation of embryos would hinder consistent, high-resolution imaging and the capturing of the same region of interest (ROI), which are crucial for accurate assessment.
To address this, scientists and Bruker’s IM team co-developed an experimental design using adapters to create indentations of agarose gel to orient embryos uniformly in 96-well microtiter plates. They generated 3D-printed molds to shape agarose gel within 96-well microtiter plates into a cavity, allowing the zebrafish larvae to align in the same direction. In addition, different geometries allow users to smoothly orient specimens either dorsally or laterally.1
The Acquifer IM’s sample-centered approach offers precision and reliability with friction-free linear motor technology and a motorized lid for robot-ready operation. Its moving optics keep the sample static, which is ideal for sensitive specimens, and built-in temperature regulation ensures optimal conditions. Other features include feedback microscopy, an open developer interface for scripting, and an optional photomanipulation module, making it a versatile tool for advanced imaging needs.
However, even with standardized body axis positioning, embryo positioning may vary between the samples, hampering standardized high-resolution imaging. To overcome this, the team created Plate-Viewer visualization software, which enables seamless annotation of regions of interest (e.g., tissues and organs) for subsequent imaging. This means that exact anatomical regions of interest can be acquired, and standardized data analysis is achieved more easily. Together, standardizing the experimental setup with the Acquifer IM, 3D orientation tool, and Plate-Viewer software, data from complex specimens can be acquired and analyzed in a high-throughput fashion.
Application Example: Automating Workflows from Sample Preparation to Results
To further develop HCS experimental design, Westhoff et al. (2020) created a workflow with several semi-automated steps, including targeted imaging of regions of interest, morphological profiling, and interactive data visualization.2 In this study, a compound library of approved drugs was tested to screen for drug nephrotoxicity. The aim was to study 1,280 compounds in over 15,000 embryos and quantitatively assess 26 parameters of phenotypic alterations. Such an endeavor exemplifies the need for automated steps.
Specimens were treated at 24 hours post fertilization (hpf) with compounds from the Prestwick Chemical Library R© (Prestwick Chemical, D’Illkirch, France). At 48 hpf, larvae were placed into an agarose gel set with 3D-printed molds to standardize larval position. The Acquifer IM was then used to acquire a Z-stack of GFP and brightfield channels, using built-in autofocus on the GFP channel to determine Z-stack centers.
Brightfield images were used for generic phenotypic observations (malformation, oedema, etc.) resulting from drug treatment, while precise manual annotation of kidney morphology was performed on GFP images. The study assessed the impact of 1,280 compounds with over 15,000 embryos. This generated 4.2 TB of data, which was stored and processed with Acquifer HIVE, Bruker’s big data management solution for centralized data storage and computing.
Automated Imaging and Processing
Semi-automatic ROI Selection with the Acquifer Plate-Viewer
Semi-automated approaches to imaging and analysis are ideal HCS approaches as they require minimal user interaction and no custom algorithm development. This approach is beneficial when working with variable data requiring extensive image detection routines.
The Acquifer Plate-Viewer uses this semi-automated approach for supervised feedback microscopy. It visualizes low-magnification pre-screen data of a full microtiter plate following the plate layout, providing a quick and intuitive overview.
The integrated Click-Tool allows microscope users to select regions of interest for each well, and built-in Template Matching algorithms robustly localize various target structures, including complex reporter expression patterns, morphological features, or rare events. Acquifer IM automatically acquires high-resolution data from selected regions based on predefined settings, intuitively visualized in the Acquifer Plate-Viewer.
Template Matching
In HCS, automated localization of objects in biomedical imaging often relies on classical image analysis methods, such as thresholding or edge detection. However, these methods often struggle when images have poor contrast or partial structures. Therefore, more generic multi-step approaches can be useful to break down a complex task into more manageable steps.
Such a multi-template matching to improve detection capacity was presented previously by Thomas and Gehrig.4 The approach aided HCS and was implemented as a Fiji plugin, KNIME workflow, and Python package. It also enhances object localization by utilizing multiple templates for easy automatic localization in microscopy images. The implementation of this method, which requires no data pre-processing or annotation, is suitable for various applications, including large-scale automated microscopy datasets and time-lapse assays.
Batch Processing with Customized Macro
Being able to quantitatively analyze data is also critical for HCS (e.g., morphological profiling after segmentation for phenotypic screening). Given the amount of data, batch processing is pivotal in HCS.
The Bruker Acquifer Fiji5 Update site is an ideal toolbox for image processing, visualization, and batch processing. The update site comes with several built-in tools, such as a plate montage viewer, and allows for image processing with Fiji Macros and other Fiji-supported languages, such as Java or Ruby.6 Furthermore, it provides a multitude of examples and detailed documentation for the development of analysis pipelines and smart microscopy workflows.
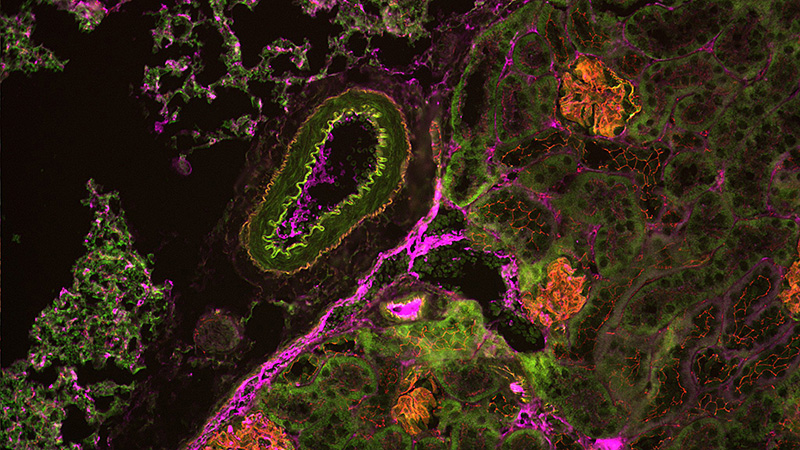
Application Example: Understanding Kidney Development and Disease from Bench-to-Bedside
Focal and segmental glomerulosclerosis (FSGS) is a group of diseases that scar the kidney’s glomeruli, leading to leakage of protein into urine. Studying FSGS causes and potential treatments is challenging due to the lack of suitable cell culture-based assays that replicate such physiological conditions as glomerular perfusion. To overcome this technical limitation, zebrafish can model recapitulating kidney diseases, utilizing the nitroreductase/metronidazole (NTR/MTZ) system.7 The NTR/MTZ system allows the induction of FSGS lesions by adding drugs to the water, avoiding individual kidney injections.
In studies from Schindler et al. (2023) at the University of Medicine Greifswald, the NTR/MTZ system was used to induce FSGS lesions and screen a library of epigenetic modulators and FDA-approved drugs to assess their impact on zebrafish kidneys.8,9 The automated analysis of thousands of zebrafish larval images required substantial storage and processing, which was achieved using Acquifer HIVE. Custom Fiji and image analysis macros enabled vasculature segmentation and the detection of podocytes via mCherry fluorescence.This high-content screening identified Belinostat as protective in an FSGS-like zebrafish model.
Application Example: Organoid Development
In organoid research, drug screens have shown promising results, exemplified by using a GSK3 inhibitor in Medaka-derived organoids.10 By inhibiting GSK3, the Wnt/beta-catenin pathway is promoted, leading to retinal pigmented epithelium (RPE) formation with specific expression of the RPE marker Otx2. This approach highlights the potential of organoid models in drug screening and development, providing insights into tissue-specific responses and the molecular mechanisms underlying organogenesis. Such advances demonstrate how organoid systems can be utilized for high-throughput drug screening, accelerating the identification of therapeutic targets and enhancing our understanding of developmental biology.
Transforming Drug Discovery
Acquifer IM and Acquifer HIVE are ideal tools for semi-automated HCS in drug discovery research. While most of the examples presented here covered zebrafish and organoids, the same approaches can be applied to other models, such as cell cultures or patient-derived tissue biopsies. Combining Acquifer IM automated widefield microscopy and Acquifer HIVE centralized data storage and computing enables efficient and effective workflows, from sample prep to final results.
Authors
Dr. Jochen Gehrig, Senior Product and Applications Specialist (Jochen.gehrig@bruker.com)
Dr. Laurent Thomas, Software Engineer (laurent.thomas@bruker.com)
Dr. Elisabeth Kugler, External Marcom Specialist (e.kugler-extern@bruker.com)
References
- J. N. Wittbrodt, U. Liebel, and J. Gehrig, “Generation of orientation tools for automated zebrafish screening assays using desktop 3D printing,” BMC Biotechnology, vol. 14, no. 1, p. 36, May 2014, doi: 10.1186/1472-6750-14-36.
- J. H. Westhoff et al., “In vivo High-Content Screening in Zebrafish for Developmental Nephrotoxicity of Approved Drugs,” Front Cell Dev Biol, vol. 8, p. 583, Jul. 2020, doi: 10.3389/fcell.2020.00583.
- J. Gehrig, G. Pandey, and J. H. Westhoff, “Zebrafish as a Model for Drug Screening in Genetic Kidney Diseases,” Front. Pediatr., vol. 6, Jun. 2018, doi: 10.3389/fped.2018.00183.
- L. Thomas and J. Gehrig, “Multi-template matching: a versatile tool for object-localization in microscopy images,” BMC Bioinformatics, vol. 21, Feb. 2020, doi: 10.1186/s12859-020- 3363-7.
- J. Schindelin et al., “Fiji - an Open Source platform for biological image analysis,” Nat Methods, vol. 9, no. 7, Jun. 2012, doi: 10.1038/nmeth.2019.
- Acquifer, ACQUIFER update site for Fiji, (Apr. 19, 2022). Accessed: Jul. 22, 2024. [Online Video]. Available: https://www.youtube.com/watch?v=eZ1cfOkkTEE
- S. Curado, D. Y. R. Stainier, and R. M. Anderson, “Nitroreductase-mediated cell/ tissue ablation in zebrafish: a spatially and temporally controlled ablation method with applications in developmental and regeneration studies,” Nat Protoc, vol. 3, no. 6, pp. 948–954, 2008, doi: 10.1038/nprot.2008.58.
- M. Schindler et al., “A Novel High-Content Screening Assay Identified Belinostat as Protective in a FSGS-Like Zebrafish Model,” J Am Soc Nephrol, vol. 34, no. 12, pp. 1977– 1990, Dec. 2023, doi: 10.1681/ASN.0000000000000235.
- M. Schindler, S.-M. Bach, J. Gehrig, and N. Endlich, “In vivo-Medikamenten-Screening zur Behandlung von Glomerulopathien,” Biospektrum, vol. 29, no. 5, pp. 494–496, Sep. 2023, doi: 10.1007/s12268-023-1997-5.
- L. Zilova, V. Weinhardt, T. Tavhelidse, C. Schlagheck, T. Thumberger, and J. Wittbrodt, “Fish primary embryonic pluripotent cells assemble into retinal tissue mirroring in vivo early eye development,” eLife, vol. 10, p. e66998, Jul. 2021, doi: 10.7554/eLife.66998.
LEARN MORE: