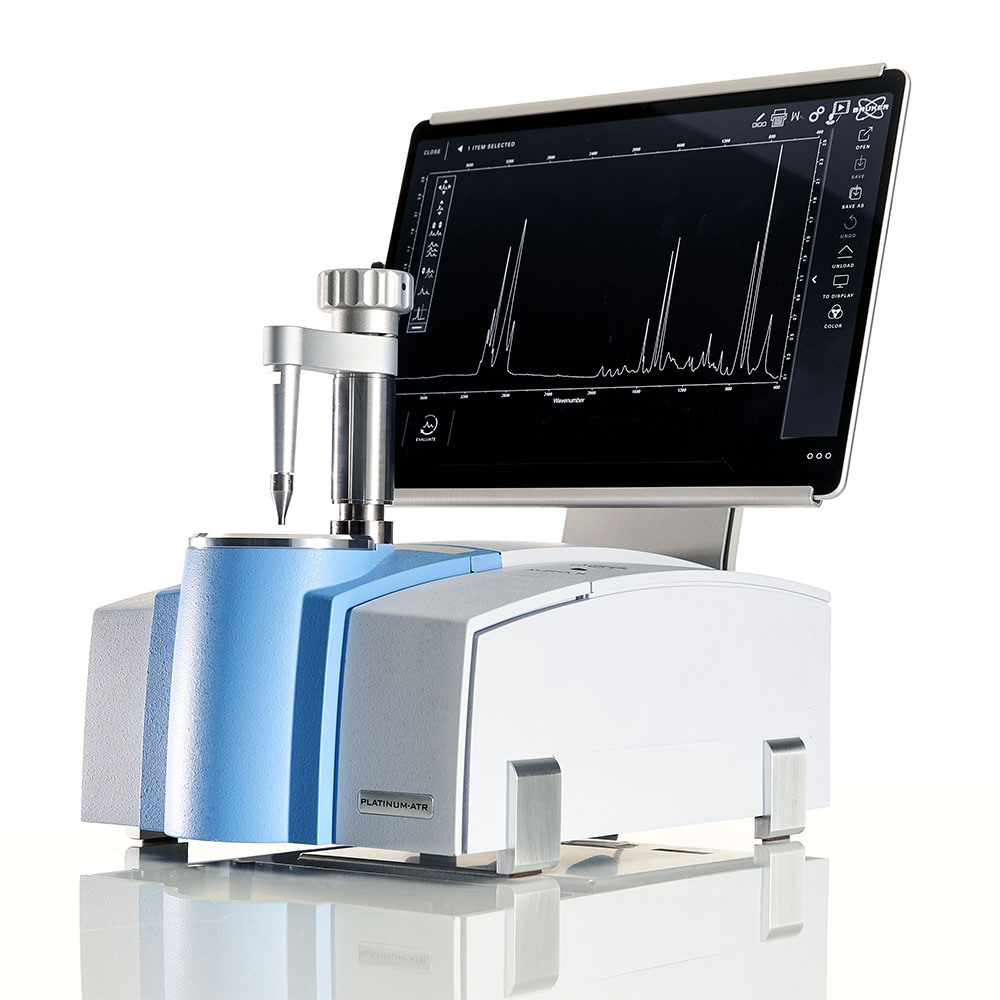
Guide to Infrared Spectroscopy
What is IR Spectroscopy?
Infrared, or IR, spectroscopy is a chemical analysis technique that takes advantage of the interaction between infrared light and matter. Infrared light is part of the electromagnetic spectrum between visible light and microwaves, with wavelengths ranging from 780 nm to 1 mm.
Due to historical reasons however, we typically discuss IR light in spectroscopy in terms of wavenumbers rather than wavelengths. Wavenumbers tell us the number of wavelengths per unit distance and are given in units of cm-1. Light with shorter wavelengths and higher energy will have a larger wavenumber, while light that has longer wavelengths will have lower energy and wavenumbers.
Mid-IR, near-IR, and far-IR
Infrared light can be further divided into three categories: Near infrared (NIR), mid infrared (MIR), and far infrared (FIR). NIR has the shortest wavelength with higher wavenumbers while FIR has the longest wavelength with lower wavenumbers.
Typically, when discussing IR spectroscopy MIR is the type of IR light that is used. The IR light in this range is useful because it coincides with an important property of chemical compounds: their vibrations.
Types of vibrations in IR spectroscopy
The atoms in chemical compounds are constantly moving and vibrating in different ways. Even in a simple molecule like water there are six different ways the molecule can vibrate: the symmetric stretch, the antisymmetric stretch, the deformation or bending vibration, rocking, twisting, and wagging.
Each of these vibrations occurs at a different frequency that is unique to the chemical bond and compound. As mentioned earlier, those frequencies happen to match the frequencies of light in the MIR region of the electromagnetic spectrum.
Detecting IR absorbtion
As these frequencies match those of the IR light, chemical compounds can absorb IR light which excites the vibrations in the molecules.
For example, the symmetric and antisymmetric stretches for water occur in the range of 2700 to 3700 cm-1 while the deformation vibration occurs around 1650 cm-1, so water will absorb those energies of IR light.
If we shine IR light through some water, we can use a detector to determine which frequencies of light were absorbed as those frequencies will be “missing” from the original beam of IR light.
Getting an IR spectrum
After the IR light is detected, we can plot the information obtained from the detector to create the IR spectrum. The spectrum shows which frequencies of light were absorbed by the sample and therefore which vibrations were excited when the IR light passed through the sample.
For the sample of water, the spectrum would show signals at the wavenumbers corresponding to the frequencies of the symmetric stretch and antisymmetric stretch, as well as the deformation vibration.
As each chemical species will have vibrations at different frequencies, the resulting spectrum of each compound will be unique. This means IR spectroscopy creates a “chemical fingerprint” that can be used to identify and quantify almost any chemical species.
Over the years, information on a vast number of chemical species has been complied into spectral libraries making IR spectroscopy extremely straightforward even for those without knowledge of the theory behind it.
What is FT-IR spectroscopy? Where is the difference to IR spectroscopy?
Though IR spectroscopy has become a somewhat general term for the chemical analysis technique where molecular vibrations are detected using IR light, the technique most frequently used is Fourier Transform IR Spectroscopy (FT-IR).
Historically, IR spectroscopy was performed by individually checking each frequency of IR light to see if it was absorbed by the sample. As you can imagine, this was a very time-consuming process!
This technique was superseded by FT-IR, which can check all the wavelengths of IR light at once using an interferometer.
This technique is not only much faster than IR spectroscopy but is also more accurate with a better signal to noise ratio. However, this technique produces a different data set than the traditional IR spectroscopy experiment so a mathematical operation, called a Fourier transform, is used to convert the data into the familiar IR spectrum obtained from IR spectroscopy.
Measurement techniques in infrared spectroscopy
There are three main measurement techniques that can be used during FT-IR spectroscopy: Transmission, Attenuated Total Reflection (ATR), and Reflection. Though all techniques rely on the underlying theory behind FT-IR spectroscopy, each technique uses a slightly different process to analyze the sample and is suitable for different purposes.
Transmission IR spectroscopy
Transmission is the “original” technique used with IR spectroscopy. During transmission detection, IR light passes through the sample where it is absorbed. For transmission to be performed, the IR light needs to completely pass through the sample which often requires the sample to be prepared in a specific way. If the sample is too thick for the IR light to pass through, too much IR light will be absorbed by the sample which is called total absorbance. Total absorbance results in poor spectral quality, with peaks that are not easily distinguished.
To avoid total absorbance, the sample is diluted which allows the IR light to pass through the sample without being absorbed too strongly. The substance that is used to dilute the sample should not absorb IR light in the same range as the sample. Otherwise, the substance used to dilute the sample would also appear on the resulting spectrum.
To analyze a liquid sample, the liquid is diluted with a solvent. The usual choice is carbon tetrachloride (CCl4). To analyze a solid sample, the solid must be ground and mixed with another solid, typically potassium bromide (KBr) which does not absorb light in the MIR range. The resulting mixture is pressed into a pellet to be analyzed. Alternatively, the sample can be very thinly sliced and placed on a KBr window. This sample preparation can only be skipped if the sample is extremely thin (< 15 µm).
The sample preparation process for transmission detection is very time consuming and requires a lot of effort. Additionally, preparing the samples this way destroys the original sample. As a result, transmission detection is only used for specific spectroscopic applications such as examining polymer films, proteins, and samples containing oil in water. However, transmission is widely used in FT-IR microscopy in the field of forensics, as well as while analyzing tissue samples and microplastics.
ATR IR spectroscopy
ATR has largely surpassed transmission and is now the primary measurement technique used as this method involves minimal sample preparation and is non-destructive. To use ATR, the sample is simply placed on top of a crystal which is typically made of diamond, germanium, or zinc selenide. The IR light is directed through the crystal where it is partially absorbed by the sample. The IR light then passes through the crystal again and is detected.
During this technique, the light only interacts with the first few microns in the sample. Since the IR light does not pass completely through the sample like in transmission, little to no sample preparation is required to create an IR spectrum using ATR. This not only results in a technique that is extremely simple to perform, but also one that produces very high-quality spectra no matter what sample is being analyzed.
It is worth knowing that ATR and transmission produce slightly different spectra due to the differences between the two techniques. These spectral differences arise due to the way different wavelengths of IR light interact with the sample when the light is only partially absorbed during ATR. However, the spectra produced by ATR and transmission are still quite similar and the difference can easily be corrected using computer software, allowing for direct comparison of spectra obtained by different measurement techniques.
Reflectance IR spectroscopy
The final measurement technique is reflection. With this technique, the IR light that is reflected off the surface of the sample is detected rather than the IR light that passes through the sample. This makes Reflection IR Spectroscopy useful for examining solid samples that are difficult or impossible to analyze with transmission or ATR. There are many ways to perform Reflection IR spectroscopy depending on the sample being analyzed.
- Reflection-absorption or “transflectance” shines IR light through a very thin sample onto a reflective substrate. This technique is useful for analyzing thin tissues or coatings.
- Specular reflection analyzes the IR light that is bounced off a reflective surface, which is useful for examining large samples like polymers, gemstones, or even pieces of art prior to restoration.
- Diffuse reflection, where light is scattered off a sample surface, is used in a technique called Diffuse Reflectance Infrared Fourier Transform Spectroscopy (DRIFTS).
DRIFTS requires careful sample preparation, so it is more difficult to perform, but it yields excellent quantitative results when analyzing solid samples such as soils, concrete, or catalysts.
Like with ATR, the differences between reflection and other measurement techniques result in different spectra due to the way the IR light interacts with the sample. The different methods of reflection detection impact the spectra differently, but all these differences can be corrected using computer software.
The many applications of (FT-)IR spectroscopy
At its core, IR spectroscopy has two main uses: identification and quantification.
IR spectroscopy has long been used for qualitative analysis as an IR spectrum is like a “chemical fingerprint” of a chemical species. This makes IR spectroscopy a powerful technique for identifying what substances are present in a sample, making it a valuable chemical identification tool not only in a laboratory setting, but in virtually any industry. FT-IR can be used for identification purposes in forensics, plastic recycling, failure analysis, quality control, and much more.
FT-IR can also be used to quantify components in a sample made of more than one substance. Individual components can absorb IR light more strongly if the concentration of that component is higher. Using computer software, the concentration of each component can be easily determined by analyzing the amount the IR light that was absorbed. FT-IR can provide extremely precise quantitative results, depending on the nature of the sample. This makes FT-IR useful for quantitative applications in areas such as the pharmaceutical industry, soil science, and biological research on proteins.
Before (FT-)IR spectroscopy came IR light discovery!
The field of IR spectroscopy originates with the discovery of IR light in the 1800s. The British-German astronomer Sir William Herschel hypothesized that different colors of light had different temperatures. He tested this hypothesis by using a prism to separate the light and checking the temperature of each color with a thermometer.
As he moved the thermometer through the colors of light from violet to red light, he noticed the temperature increased. Then he noticed something curious. When he placed the thermometer just beyond the red light, the temperature rose even more. He proposed that there was another form of invisible light beyond the red light, which he called “calorific rays” (from the latin word “calor,” meaning heat). Further experimentation proved that this was exactly right, he had discovered a new form of light we now call infrared light.
In the early 1900s, William Weber Coblentz discovered that IR light interacts with matter and realized the potential of using IR light in chemical analysis. Coblentz created the first IR spectra and gathered the necessary data to characterize a wide range of compounds. There were several initial challenges with creating a commercially viable IR spectrometer, but one eventually came to market in the 1940s. IR spectroscopy was continuously improved throughout the 1900s, with FT-IR revolutionizing the technique around 1970, and ATR improving it again in the late 1980s.
Though IR spectroscopy has been around for a long time now, it’s still an incredibly powerful chemical analysis technique with new uses and advances being implemented every year. For example, recent developments in MIR laser technology allow IR spectroscopy to be easily applied to entirely new fields of study, such as investigating structural changes of biological molecules. IR spectroscopy has also been coupled with dozens of other techniques making its impact as a chemical analysis tool even more widespread. The ease of use and wide-ranging applications of IR spectroscopy ensure that it will continue to find new and exciting applications in the years to come.
IR light in other techniques
IR spectroscopy isn’t the only way we can make use of IR light and the principles behind FT-IR. There are also FT-IR microscopes that combine traditional microscope technology with FT-IR spectroscopy, creating a powerful characterization and imaging technique.
Raman Spectroscopy is another chemical analysis technique which takes advantage of chemical vibrations in a very different way, providing detailed information on the structure of analyzed samples. The use of IR lasers is a recent addition to IR spectroscopy, allowing detailed analytical spectroscopy to be performed which can give insight into new research areas.
FT-IR basics tutorial videos
Application of FT-IR spectroscopy
Frequently asked questions about FT-IR
What is infrared light?
Infrared (IR) light, or more precisely infrared radiation, is an electromagnetic radiation (EMR) with wavelengths longer than those of visible light. It is therefore invisible to the human eye but can be perceived in the form of thermal radiation. Fun fact: more than half of the energy radiated by the sun reaches the earth in the form of infrared.
How does infrared light interact with materials?
When infrared radiation is directed at matter, it can stimulate the movement of molecules and atomic bonds. This movement can take various forms, such as rotation or vibration. Depending on how the molecule is excited, we can obtain information about the structure and identity of the irradiated material.
Can infrared light analyze all materials?
In general, yes, because organic and inorganic substances can be examined equally well with infrared radiation. The basic requirement for analysis with infrared is that the material absorbs infrared radiation. Certain substances, however, including metals and monatomic gases (e.g. noble gases) cannot be examined directly.
Which materials are commonly analyzed?
Especially for organic substances IR spectroscopy is a frequently used tool to obtain a lot of information. This includes the identification of polymers, drugs, pharmaceuticals, or industrial chemicals as well as the determination of contents like water in oil. IR spectroscopy is very flexible, and its applications are so numerous that you can find IR users in all industry and research areas.
What kind of analysis is possible?
With IR it is possible to find out what sample is made of, but also how much of a certain ingredient or component is present. Qualitative analysis is the most common application of IR spectroscopy and is mainly used in quality control of raw materials, failure analysis, and in scientific research. Quantitative analysis is widely used in industrial processes to evaluate production parameters.
Do I need to be an expert to use IR spectroscopy?
Definitely not. IR spectrometers are easier to use today than ever before. Most of the time there are simple software solutions (e.g. touch operation) that allow non-experts to perform IR analyses in an uncomplicated way. Even the analysis can be automated, so anyone can become a spectroscopist!
How long does IR analysis take?
This depends strongly on the analytical question posed. But a simple verification of the identity of a chemical substance hardly takes more than a minute.
What is attenuated total reflectance (ATR)?
ATR is a special sampling technique to obtain IR information. The IR light is directed at a crystal made from IR transparent material (e.g. diamond). The IR radiation will then interact with samples and materials that are in close contact with the diamond. Watch our video on ATR basics to learn more!
Where do I use ATR?
Almost everywhere, as ATR is a truly universal approach. Whether solid or liquid, organic or inorganic - you simply take your sample and place it on top of the crystal. There is no need to cut, dilute or prepare your sample. In the last decades, ATR has become the standard technique in IR spectroscopy.
What is transmission?
Different to ATR, this method requires light to penetrate the whole sample. This means that the sample must be either very thin or diluted. For dilution, samples are often mixed with potassium bromide (KBr) and pressed into a pellet. Very thin samples, on the other hand, are produced with a microtome and then placed on a KBr window. These preparations require a lot of time and effort.
When do I use transmission?
Today transmission is only necessary for very specific analytical questions. These include the quantification of lowly concentrated components in liquids or the application IR microscopy. In certain industrial sectors there are also standardized procedures that require transmission measurements (e.g. Pharma).
What is reflectance?
Reflection is the third main technique in IR spectroscopy. It is based on the reflection of IR light and allows conclusions about the surface of materials. If the surface in question cannot be examined directly, dilution with KBr is often necessary. It is also possible to place very thin samples on metallic mirrors (transflectance).
Where is reflectance used?
Due to the special requirements of reflectance measurements, it is used for very specific analytical objectives. It is possible, for example, to examine valuable works of art completely non-destructively and carefully to enable their restoration.