Life Sciences Research AFMs
Advanced Imaging
Bruker has been helping biologists discover, understand, publish, and advance their knowledge of biological systems ever since our introduction of TappingMode™ expanded AFM use into life sciences research. In addition to general-purpose BioAFM systems specifically designed for life sciences research and suitable for a broad range of applications, Bruker offers a variety of specialized systems utilizing proprietary AFM features. These instruments include BioAFMs for high-speed imaging to support the investigation of dynamic processes; BioAFMs optimized for the investigation of cells and tissues, even uneven or highly corrugated samples; and BioAFMs for single-molecule applications ranging from single-molecule property characterization to the investigation of conformational changes at the single-molecule level, intra-molecular dynamics, and single-molecule spectroscopy (SMFS).
These instruments deliver the highest quality results for quantitative live-cell mechanical property mapping, highest resolution molecular and cellular imaging, and fast scanning of dynamic biological processes, as well as seamless integration of AFM with advanced optical microscopy techniques.
Find the Best BioAFM for Your Application
Customize Your BioAFM System
Accessories to extend your BioAFM capabilities
Bruker offers an extensive range of BioAFM system add-ons, accessories, and modes to deliver maximum experimental and sample control, superior versatility, and enhanced useability. These options extend the range of applications and experiments supported by Bruker BioAFMs far beyond what is possible with any other BioAFM system on the market today.
Available options include optical systems/accessories, electrochemistry solutions, electrical sample characterization, environmental control options, software modules, temperature control, acoustic and vibration isolation solutions, and more.
Browse our online accessories database or download our accessories handbook to learn more.
Explore Our Virtual Showroom
Frequently Asked Questions
BioAFM is an increasingly important tool in biological and biomedical studies due to its very high resolution and the possibility of conducting experiments with live cells under physiologically relevant and ambient conditions, even in liquid. BioAFM also provides nanometer-resolution surface mapping for many mechanical and electrical properties, such as elasticity, stiffness, conductivity and surface potential. Bruker BioAFM technology is enabling life science researchers to investigate how these properties impact on cellular functions, including communication, signaling, cell division and differentiation, and even tumor metastasis and infection.
What is a BioAFM?
A BioAFM is an atomic force microscope specifically adapted for studying biological samples and soft matter. BioAFM instruments can measure soft, fragile, and challenging samples — including single molecules, nucleic acids, bacteria, living cells, and tissues — under near-physiological conditions without damaging their structures. These instruments are uniquely capable of performing non-invasive measurements on biological samples and label-free measurements in liquid.
How are BioAFMs specifically adapted for biological studies?
BioAFMs have specialized sample and cantilever holders, stages, and measurement modes specifically designed for life science samples and experiments. Bruker BioAFM instruments also can be uniquely configured with a large selection of optional accessories and advanced modes, extending sample compatibility and measurement capabilities to include even the most challenging soft matter and biological samples.
Unique features and capabilities of Bruker BioAFM instruments include:
- High-speed AFM with up to 50 frames per second enables the visualization of molecular dynamics.
- Precise force control prevents damage of delicate biological samples.
- Fully integrated correlated analysis with a wide range of advanced optical techniques.
- Data analysis methods for soft samples (e.g., contact point imaging).
- Large z range (>100µm) for measuring challenging biological samples with steep, highly corrugated surfaces, and cell adhesion experiments.
- Advanced automation features (e.g., detector alignment, creation of experiment workflows, and adjustment of scanning parameters) enable self-regulating measurement routines that maximize throughput and improve accuracy.
- Optical Tiling software feature for navigating around large samples.
- Cantilever holders that can be cleaned and autoclaved, providing direct access to biohazards in biosafety laboratories and allowing sample preparation, loading, experiments, and disposal to be performed in the BSL facility.
- Accessories for investigating a diverse range of biological and soft matter samples on substrates of various sizes, shapes, and materials (e.g., Petri dishes, coverslips, biochips, etc.) and in different environmental conditions (e.g., atmosphere, temperature, gas, pH-value, etc.).
- Automated, large area, multiparametric characterization of densely packed cell layers and highly corrugated tissue samples without microtome cutting.
What are the advantages of using a BioAFM?
Atomic force microscopy provides 3D images of surface features and topography. BioAFMs extend and enhance the technique, optimizing it to address the unique needs and challenges of biological research and providing distinct advantages over other methods. Key advantages include:
- High spatiotemporal resolution: Investigate morphology/surface structure at sub-molecular resolution and interactions in the piconewton range.
- AFM in liquids and environmental control: Study samples in liquid and under near-physiological conditions — ideal for the investigation of living cells in medium — capabilities that are unique to atomic force microscopy.
- Label-free and non-invasive investigation of living cells and delicate biological samples.
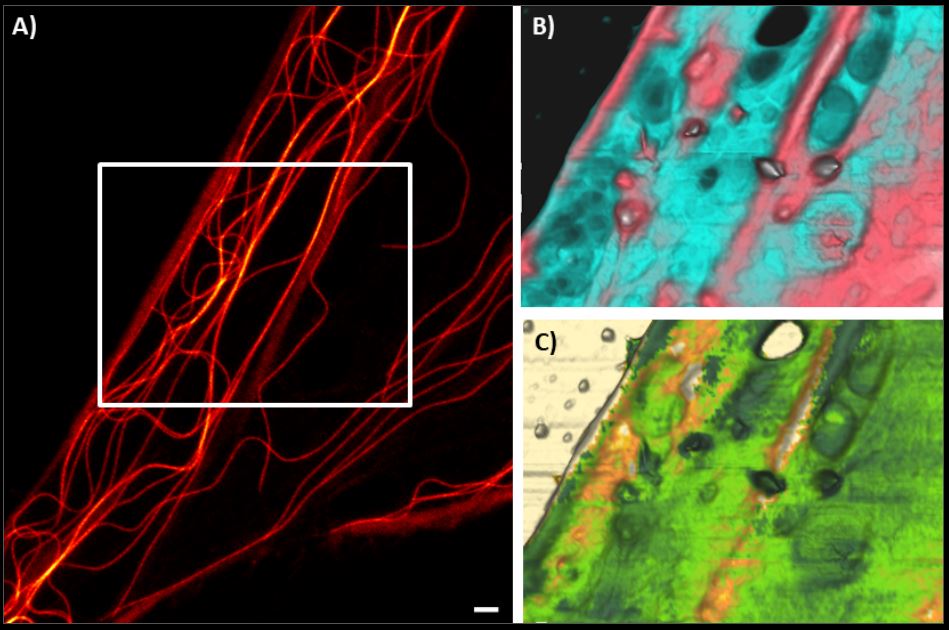
- Easy combination with other advanced optical microscopy techniques for correlated measurements and complementary datasets that provide a more comprehensive insight into biological processes.
What are the advantages of automated BioAFM measurements?
Bruker BioAFMs feature several specialized software functions and capabilities designed to support high-performance AFM-based investigation of biological samples and soft matter. Automated instrument procedures (e.g., alignment, operation, calibration), measurement routines, and data analysis capabilities make it easier to set up and run experiments while also improving the accuracy and reproducibility of results.
Advanced automation features include:
- ExperimentPlanner: Predefinition of settings and parameters allows complex experiments to run automatically.
- ExperimentControl: Remote monitoring of long-term lab experiments via a browser on any device. Together with ExperimentPlanner, these features open a host of new possibilities for long-term, self-regulating experiment series that reflect “real-life” conditions. This alleviates long and repetitive experiments and gives researchers the freedom to oversee and control their experiments remotely.
- SmartMapping: The novel SmartMapping feature allows the flexible selection of multiple, user-defined 2D force maps. Numerous regions of interest (ROI) can be selected in advance and examined automatically, enabling the systematic study of large sample areas.
- Automated large-area, multi-region imaging (by combining DirectTiling, DirectOverlay 2, and MultiScan software features) extends the optical viewing field. Perfect optical integration enables direct correlation of AFM and optical data.
These features extend and enhance the capabilities of Bruker BioAFM systems in order to:
- Increase throughput and productivity.
- Produce statistically relevant datasets.
- Reveal parameter correlation and cross-correlation via automated cycling through of parameters.
- Allow unattended, long term, self-regulating experiment series.
- Enable remote monitoring of long-term lab experiments.
The improved throughput, standardized batch analysis routines, and statistically relevant datasets generated by these types of automated features are essential in biological research, particularly in nanomedical and clinical fields.
How and why do you integrate a BioAFM with an optical/fluorescence microscope?
A major advantage of Bruker’s BioAFM instrumentation is that they can be easily combined with advanced optical microscopy techniques, e.g., fluorescence or STED microscopy, for correlated measurements and complementary datasets.
Our BioAFMs can be easily combined with advanced optical imaging techniques by using a specific AFM stage that is compatible with most commercially available inverse and confocal optical microscopes. The AFM head is placed on the stage and software, such as Bruker’s DirectOverlay feature, enables colocalization of the optical and AFM images and direct correlation of the AFM and optical data. Transferring the sample between setups is not necessary and a wide range of camera and detector types are supported. Easy optical image import, advanced calibration algorithms, and visualization routines facilitate accurate navigation on and around the sample, providing multidimensional sample characterization in a single experiment. Compatible techniques include epifluorescence, confocal, phase contrast, and super-resolution microscopy techniques (STED, TIRF, STORM).
The ability to obtain real-time, correlative data sets is particularly relevant in life science research because it:
- Allows researchers to study the topography of biological samples using AFM while simultaneously observing fluorescently labeled cellular components; and
- Enables the multiparametric observation of in-situ dynamics, such as single-molecule protein dynamics, protein folding, receptor-ligand interactions, and mechanosensitive signaling pathways.
RELATED LINKS:
- [WATCH] High-Speed AFM: Fundamentals, Applications, and Integration with Advanced Optical Techniques
- [WATCH] Correlative Optical Microscopy and AFM: Enhancing Biological Studies
- Browse Our Application and Technical Notes on Integrated Optical Techniques
- BioAFM Applications Gallery: AFM/OT and Advanced Optics
Is an optical microscope required to use a BioAFM instrument?
No, you do not need an optical/upright microscope for BioAFM-based experiments; a BioAFM instrument can operate independently of any other microscope system.
What Can You Do With a BioAFM?
What can be studied with a BioAFM?
BioAFM allows the study of:
- Morphology: Accurate force control and precise movements allow the scanning of surface morphologies with extreme accuracy.
- Dynamics: Highest scanning speeds enable the real-time visualization of dynamic biological processes as well as phase and structural transitions.
- Nanomechanics: Specialized BioAFM instrumentation enables label-free, multiparametric characterization of nanoscale biomechanical properties, such as Young’s Modulus or unbinding forces.
- Microrheology and viscoelastic properties: Highly reproducible force curves and microrheological measurements enable the study of viscoelastic properties.
- Structure: Automated long-term measurement routines and hardware allow the study of the structure and dynamics of protein folding and unfolding events.
- Measurement of interaction forces at the cellular level: Cell-cell and cell-substrate adhesion can be quantified using single-cell force spectroscopy and large piezos.
RELATED LINKS:
- Browse Our BioAFM Resource Library
- Browse BioAFM Webinars
- BioAFM Applications Gallery: AFM/OT and Advanced Optics
- BioAFM Applications Gallery: Cell Mechanics and Adhesion
- BioAFM Applications Gallery: Electrical, Magnetic, and Thermal Measurements
- BioAFM Applications Gallery: High-Speed Imaging
- BioAFM Applications Gallery: Life Science Research
- BioAFM Applications Gallery: Nanomanipulation & Nanolithography
- BioAFM Applications Gallery: Nanoscience
- BioAFM Applications Gallery: Raman, TERS, and SNOM
What type of research are BioAFMs used for?
Bruker BioAFMs achieve industry-leading precision and repeatability in the above-listed applications and support advanced and cutting-edge research in cancer research, polymer research, biomaterials for use in medical implants and tissue regeneration, and microbial interface biology. Further examples include:
- Cell Biology: BioAFM is used to study the structure and morphology of cells, membranes, and surface features, as well as the mechanical properties of cells, such as stiffness and elasticity;
- Molecular Biology: BioAFM can visualize the structure and dynamics of individual proteins and protein complexes, providing insights into conformational changes and interactions at the molecular level;
- Developmental biology: BioAFM can be used to image and analyze the composition and organization of the extracellular matrix (ECM) during development, providing insights into how the ECM supports and regulates cell behavior.
- Nanomedicine: The BioAFM-based investigation of the size, surface, and structural properties of nanoparticles, pharmaceutical products, and drug delivery systems reveals how these parameters influence the bioavailability and efficacy of active ingredients. BioAFM also enables the identification of morphological changes occurring following contact of a drug candidate with a cell membrane and quantification of cell-surface interactions.
- Development of Biomarkers: Many pathological diseases, such as cancer and osteoarthritis, are closely associated with changes in the mechanical properties of cells, such as stiffness and elasticity. BioAFMs can be used to investigate these properties, facilitating their use as biomarkers for the early detection of an underlying disease.
- Microbial Research: BioAFM can be used to image the cell walls and surface structures of individual bacteria. It is also used to study the formation and mechanical properties of biofilms, important aspects in understanding bacterial populations and their resistance to antibiotics.
- Neuroscience: BioAFM can image neurons and measure the mechanical properties of neural tissues, providing crucial insights into neural network formation and function.
What are the most common applications of BioAFM systems?
The most common applications of BioAFMs are nanoscale structural analysis and biomechanical characterization. These provide valuable insights into molecular, single-cell, and cellular mechanisms and functionality. Atomic force microscopy enables the high-resolution imaging and precise measurement of the nanoscale forces necessary for these types of investigation, making it an essential tool for:
- Investigating biophysics and biomechanics (cell mechanics and adhesion).
- Mechanobiology, mechanotransduction, and the study of mechanosensitive signaling pathways.
- Structural analysis of molecules and superstructures of the cell membrane, cell morphology, and cell skeletons at the nanometer scale.
- Live cell imaging.
- Investigation of DNA and RNA binding proteins, molecular scaffolds, and nucleic acid-protein interactions.
- Studying receptor-ligand interactions, specifically the quantification of binding-affinity, cell-cell and cell-surface interactions, and cellular response.
- Single-molecule force spectroscopy (SMFS).
RELATED LINKS:
What sample types can be measured with a BioAFM?
BioAFM instruments can measure soft, fragile, and challenging samples ranging from single molecules, nucleic acids, and proteins to viruses, bacteria, living cells, and tissues. Soft matter — such as hydrogels, spheroids, organoids, and biomaterials — can also be studied in a non-invasive manner using a BioAFM.
Bruker BioAFMs can be equipped with a wide variety of accessories to facilitate the investigation of samples of varying size and composition, on a wide range of substrates, and under ambient, extreme, and/or aggressive environmental conditions.
What properties can a BioAFM measure?
A BioAFM can measure nanoscale mechanical properties (e.g., stiffness, elasticity, adhesion, Young’s modulus, dissipation, and deformation), particle size, surface structure, and morphology.
These properties can be investigated in samples as diverse as single molecules, live cells, tissues, proteins, and bacteria, as well as in soft matter samples, such as polymers and hydrogels.
What sample preparation is needed for using BioAFM?
BioAFM is a label-free method. Measurements can be performed in air or liquid, enabling the investigation of live cells under near-physiological conditions; it does not require a vacuum and it is not necessary to freeze, dry, coat, or microtome cut samples before measurement.
In general, the sample must adhere to a surface substrate (e.g., a Petri dish, coverslip, or mica), or, if measuring in liquid, be immersed in a suitable buffer solution.
It is recommended that the substrate is thoroughly cleaned before measurement to remove any contaminants or artifacts that might interfere with imaging.
Contact our BioAFM experts to discuss your specific sample and measurement requirements.
Learn More About Our BioAFM Technology
Bruker has deep experience and expertise supporting life science and biophysics research in the fields of cell mechanics and adhesion, mechanobiology, cell-cell and cell-surface interactions, cell dynamics, and cell morphology.
We are eager to share this knowledge with the larger research community. Be sure to explore our extensive offering of BioAFM resources, below.
Contact us to discuss your specific application requirements and measurement needs with a BioAFM expert.
Watch Our BioAFM Webinars
Our webinars cover best practices, introduce new products, provide quick solutions to tricky questions, and offer ideas for new applications, modes, or techniques.
How Can We Help?
Bruker partners with our customers to solve real-world application issues. We develop next-generation technologies and help customers select the right system and accessories. This partnership continues through training and extended service, long after the tools are sold.
Our highly trained team of support engineers, application scientists and subject-matter experts are wholly dedicated to maximizing your productivity with system service and upgrades, as well as application support and training.